Hypertension (HTN) is the most prevalent vascular disease in the world; an estimated 1 billion adults have it.[1] Cardiovascular disease (CVD) risk increases exponentially as blood pressure (BP) increases[2], so it is important to consider BP management as a part of overall heart health. There are many different ways to address blood pressure within a Whole Health framework. Recognizing that detailed guidelines for medical management are widely available, this tool will briefly summarize the latest professional care guidelines for HTN management and then focus in greater detail on research findings related to self-care options and complementary and integrative health (CIH) approaches. There are many options to manage BP that can be woven into Personal Health Plans (PHPs).
Blood Pressure Guidelines
The American College of Cardiology (ACC) and American Heart Association (AHA) Guidelines for 2019, which focus on prevention of heart disease in general, emphasize the following, which are consistent in many ways with a Whole Health approach[3]:
- The most important way to prevent CVD and other heart problems is through …a healthy lifestyle throughout life.
- Team-based care is an effective strategy for prevention. The guidelines suggest that Clinicians should evaluate the social determinants of health that affect individuals to inform treatment decisions. This ties in closely with Me at the center of the Circle of Health tailoring care to each individual based on their context. It also links to the Community circle, which is inclusive of social determinants and their influence on health and disease.
- Adults from 40-75 being evaluated for CVD prevention should have their 10-year risk calculated and be assessed for other risk-enhancing factors to determine whether medications should be started.
- All adults should consume a healthy diet (as described in the Food and Drink section, below). A healthy weight is key to preventing heart disease.
- As outlined in the Moving the Body section below, it is good to engage in 150 minutes weekly of moderate-intensity or 75 minutes weekly of vigorous-intensity activity.
- Treat people with type 2 diabetes with lifestyle changes, and start with metformin (or other diabetes medications when needed) if medications are indicated. Always check on tobacco use; do not typically use aspirin. Treat lipid problems with statins.
- Nonpharmacological interventions are recommended for all adults with elevated BP or HTN (in addition to medications, as appropriate).
Within that framework, then, there are ACC/AHA Guidelines specific to HTN. These focus more on specific BP parameters and, to a large degree, medication guidelines. Highlights include the following[4]:
- Accurate BP measurement is critical. Use an average of measurements from at least two different occasions. Home monitoring is associated with lower BP criteria to guide treatment. Normal BP is defined as <120/<180 mmHg. Categories go up from there; stage 2 HTN is >140 systolic blood pressure (SBP) or >90 diastolic blood pressure (DBP).
- Risk increases in a log-linear fashion. For every 20 mm Hg in SBP from 115 to 180 mm Hg, or for every 10 mm Hg increase in DBP from 75 to 115 mmHg, risk of death from stroke, heart disease, or vascular disease doubles. For 45-year olds without HTN, 40-year risk for eventually developing it is …93% for African Americans, 92% for Hispanics, 86% for Whites, and 84% for Chinese adults…
- Think of HTN in the framework of other CVD risk factors, including smoking, diabetes, dyslipidemia, excess weight, poor fitness levels, poor diet, stress, and sleep apnea. Various laboratory tests and other studies may be helpful. (That is, consider the entire person and how BP fits in to the bigger picture.)
- Criteria are given in the guidelines for how to screen for secondary causes, based on the presence of drug resistance, rapid onset, target organ damage, etc.
- Nonpharmacologic interventions are mentioned, including physical activity, limiting alcohol, weight loss, sodium restriction, potassium supplementation.
- Parameters are provided to determine at what level of CVD risk a person should be treated with medications. For example, it is recommended that adults with stage 2 HTN should be evaluated within a month, treated with non-drug therapy and 2 BP medications from different classes, and followed up a month later.
- Many other guidelines are available, based on individual circumstances (race, comorbidities, and others). More details can be found in the ACC/AHA online resources.
Me at the Center
In addition to coronary artery disease, HTN is also associated with higher risks of other heart and vascular diseases, including of heart failure, atrial fibrillation, aortic valve disease, sick sinus syndrome, and abdominal aortic aneurysms.[5] HTN is associated with non-cardiac comorbidities as well. A 2017 review of 29 articles concluded that hypertension has a significant association with breast cancer, especially for postmenopausal women.[6] There is also an association, in epidemiologic studies, with renal cancer (2-4 times the risk), colorectal cancer, malignant melanoma, and prostate cancer.[7] People with depression have an increased risk for HTN (odds ratio 1.3 for men and 1.04 for women), as do people with anxiety (odds ratio 1.3 for men and 1.05 for women).[8] A diagnosis of PTSD, and the chronicity of PTSD, are closely linked to increased risk of HTN as well.[9]
While self-monitoring can be an important way to mobilize individual efforts to manage BP, large studies have concluded that it does not improve BP or control unless it is accompanied by co-interventions with medical team members, like pharmacists, providers, or people who can provide education and/or lifestyle counseling.[10]
One significant challenge with care of people with HTN is adherence to medications. A review and meta-analysis including over 12,600 people found that 45% of people with HTN alone and 31% of people who had HTN and other health concerns were not taking their medications.[11] Two-thirds of the nonadherence was noted in people of African or Asian descent.
An individuals genetic profile can also have an influence on BP. For example, certain mutations affecting chloride reabsorption by the kidneys can cause a person to have relatively lower BPs. Polymorphisms related to angiotensin-converting enzyme (ACE) can also have an effect, particularly around how weight change affects BP.[12] Race and ethnicity also have an influence. For example, studies indicate that African Americans have more of a BP reduction than Whites when they reduce sodium, increase potassium, or follow a DASH diet.[13]
The next sections review various self-care and therapeutic approaches that can benefit BP. Note the average effects they can have on numbers; most of them lower SBP and DBO by somewhere between 2 and 6 mmHg. Think about how you can provide patients with an array of options, so that they can find the best combination of approaches that works for them. In addition to an appropriate exploration around medications, which should be kept as a high priority, what other options would you feel comfortable discussing with people?
Lifestyle
Changes in lifestyle habits can be effective for lowering BP, particularly in those who are younger and have moderately elevated readings of greater than 140/90.[14] Of course, one of the best things about working with lifestyle changes is that they have multiple benefits; they do not only improve BP readings. Exercising, eating a diet rich in fruits and vegetables, and maintaining appropriate weight also significantly reduce the risk of cancer, diabetes, and stroke
The ACC/AHA guidelines point out that each lifestyle change a person makes can decrease SBP by 4-5 mmHg and DBP by 2-4 mmHg; they also note that a diet low in sodium, and saturated/total fats and high in vegetables, fruits, and grains may decrease SBP by 11 mmHg.[15] The U.S. Preventive Services Task Force has noted that there are small but significant improvements in BP (drops of 1 mmHg or so) associated with people choosing healthy a healthier lifestyle, even without their receiving formal counseling based on risk.[16] That said, more intensive counseling is associated with greater improvements.
Table 1. Review of Lifestyle Influences on Blood Pressure[2][17][18][19][20]
| Element | Blood Pressure Reduction | Notes |
|---|---|---|
| Weight loss if overweight | SBP: 4.4 DBP: 3.6 | Data is for people with an average weight loss of 5 kg (11 lb). More weight loss means more improvements. For more information: https://www.nhlbi.nih.gov/health/educational/lose_wt/recommen.htm |
| Physical Activity | SBP 2.5 DBP 2.3 | This is after 30-40 minutes daily, most days of the week, over 12 weeks or more. Many studies find even greater reductions. |
| DASH diet | SBP 5.5 DBP 3 | Blood pressure effects were seen in many studies within just two weeks on the DASH diet. http://www.nhlbi.nih.gov/health/health-topics/topics/dash/ |
| Sodium (Na) Restriction | Decrease intake by 2.3 grams/day led to SBP drop of 3.8. Decrease by 4.5 grams/day led to SBP drop of 23 and DBP drop of 9. | Limit salt to less than 2,400 mg/day (1tsp). The body only needs 500 mg (1/4 tsp) daily. For more information on lowering salt in the diet, go to: http://www.nhlbi.nih.gov/files/docs/resources/heart/hbp_salt.pdf |
| Alcohol Restriction | SBP 3.3 DBP 2.0 Dose dependent. | Numbers are based on dropping consumption by an average of 76%. Limit to no more than 2 drinks/day for men and 1 drink/day for women. |
| Tobacco Cessation | Highly variable | Avoid first and secondhand smoke and all tobacco products. |
| Vegetarian Diets | SBP 6.9 DBP 4.7 | Based on 2014 meta-analysis. |
SBP=Systolic Blood Pressure; DBP=Diastolic Blood Pressure; gm=grams; kg=kilograms. All units are millimeters mercury (mmHg), DASH Dietary Approaches to Stop Hypertension
Food and Drink
A 2018 meta-analysis of prospective studies summarized the research related to 12 different food groups/categories and their effects on relative risk (RR) of someone developing HTN. While noting that higher-quality evidence is needed[21], they concluded that HTN risk decreased when people ate the following:
- Whole grains, 30 gm/day (RR=0.92)
- Fruits, 100 gm/day (RR=0.97)
- Nuts, 28 gm/day (RR=0.7)
- Dairy, 200 gm/day (RR=0.95).
There were also foods that increased relative risk of developing HTN:
- Red meat, 100 gm/day (RR=1.14)
- Processed meat, 50 gm/day (RR=1.12)
- Sugar-sweetened beverages, 250 ml/day (RR=1.07)
Of note, BP reduction through changes in diet seem to be even more significant in the elderly.
Vegetarian Diets
Fruits and vegetables are high in electrolytes, polyphenols, and other compounds that can favorably affect BP. Findings from a several meta-analyses point to lower BP levels associated with following a vegetarian diet[17][18], as noted in Table 1, above.
Dietary Approaches to Stop Hypertension (DASH)
A 2016 review and meta-analysis focused on the relative effects of a variety of dietary patterns on BP. Diets like the DASH diet, Nordic diet, and Mediterranean diet significantly lowered SBP by an average of 4.3 mmHg and DBP by 2.4 mmHg.[22] The Mediterranean diet seems to have more limited effects (SBP and DBP reductions of about 2 mmHg) compared to the DASH Diet. A 2019 meta-analysis of 67 trials that looked at these diets as well as a variety of others concluded, The DASH, Mediterranean, low-carbohydrate, Paleolithic, high-protein, low glycemic index, low-sodium, and low-fat dietary approaches were significantly more effective in lowering SBP (-8.73 to -2.32 mmHg) and DBP (-4.85 to -1.27 mmHg) compared to the control diet.[23] Of all these, DASH was ranked the most effective dietary approach of all.
In actuality, the DASH diet shares a lot of recommendations in common with the Mediterranean diet. It consists of foods that are:
- High in fruits and vegetables
- Low in dairy, animal meat, and saturated fat
- High in nuts, seeds, and beans
- Low in snacks and sweets.
The DASH diet also emphasizes a reduction in sodium intake and avoidance of sugar-sweetened beverages.
The DASH diet is low in saturated fat, cholesterol, and simple sugars, which can worsen inflammation and increase the risk of diabetes and heart disease. It incorporates protein more from plant versus animal sources.
In those with high BP, the DASH diet on average lowers the SBP 11.6 points and the DBP 5.3 points.[24] It also lowers homocysteine levels,[25] has a positive effect on bone strength,[26] and raises HDL by 21%-33%.[27] Although the DASH diet is an effective intervention and generally considered to represent a satisfying way to eat, program adherence is not always optimal.[28]
The DASH Diet Whole Health tool has more detailed information.
Sodium Restriction
Considerable debate continues about what are healthy upper limits for sodium intake.[29] The body only needs 500 mg daily for optimal functioning (1/4 tsp). Table 1, above, highlights reductions in BP that occur based on amounts of sodium people cut out of their diets. Reducing sodium is beneficial to African Americans as well as (though less for) Whites, and it is beneficial for both men and women.[17] Reducing sodium intake can also prevent HTN from ever occurring (relative risk reduction of 20%). It can also reduce BP even more when it is combined with medications versus when people take medications only.[30]
Direct patients to take the salt shaker off the table and avoid salt-rich foods. Foods that tend to be high in salt include canned soups, broths, frozen dinners, chips, lunch meats, salad dressings, pizza, packaged mixes, and foods eaten away from home.
For more information on a low-salt diet, refer to Implementing Recommendations for Dietary Salt Reduction.
Increased Potassium Intake
Data from individual trials have been inconsistent, but several meta-analyses now support a relationship between potassium (K) intake and better BP readings in people who have HTN; potassium does not clearly affect BPs in people with normal BP levels.[17][31] Potassium is best-obtained through the diet, and a DASH diet offers a good amount. High-potassium foods include avocados, acorn squash, apricots, bananas, broccoli, cucumbers, eggplant, grapefruit, leafy greens, mushrooms, oranges, peas, prunes, pumpkins, salmon, spinach, sweet potatoes, tomato juice, white beans, and zucchini. Milk and yogurt are also high in potassium. The DASH diet provides 4.7 gm of K per day.
Potassium has more of a favorable effect for people with high sodium intake. It also has a more substantial effect in Black people. It is suggested that people keep the ratio of K to sodium that they eat in the range of 4 or 5 to 1.[15] If people have poor kidney function, they should keep potassium intake from their diets below 4.7 gm/day (perhaps lower for more severe chronic kidney disease).[32] Many BP medications can raise or lower potassium levels.
Magnesium
Magnesium (Mg) also plays a role in BP management.[33] According to some trials, Mg can decrease SBP by 3-4 and DBP by 2.5 mm Hg, on average. The improvements are greater when a persons intake over 370 mg/day. The optimal dose of an Mg supplement seems to be 500-1,000 mg/day, and chelating it with amino acids like taurine seems to enhance its effect and improve its tolerability.[34]
Omega-3 Polyunsaturated Fatty Acids
Eating foods containing omega-3 has been associated with lower BPs. Omega-3s may work by many mechanisms, including release of nitric oxide, increasing vasodilator prostanoids, reducing insulin resistance, and stimulating the parasympathetic nervous system.[35] In people with untreated HTN, taking an average of 4 gm (a high dose) of omega-3s led to reductions of SBP by 4.5 and DBP by 3.1 mmHg.[36] Omega-3s are likely to be more beneficial for reducing the risk of heart disease through their anti-platelet and anti-inflammatory effects than by their effect on BP.
Fish oil consists of two essential fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Evidence suggests that DHA has more of a hypotensive effect than EPA.[37] Patients can use fish oil at a dose of 1 gram daily to reduce the risk of heart disease, particularly if they already have had a documented heart attack.[38][39] A 2014 review of randomized controlled trials using both EPA and DHA, however, found the combination results in reduced SBP, with higher intakes (greater than or equal to 2 gm daily) needed to reduce DBP more.[40] Nuts and flaxseed oil, as omega-3 sources, have not been studied to the same extent as fish oil.[36]
Olive Oil
Intake of extra-virgin olive oil has been found to be associated with lower SBP and DBP readings, in people eating a Mediterranean diet.[41] Identical twins with HTN, treated with 500 or 1,000 mg of olive leaf extract daily for 8 weeks, had a decrease of SBP of 3 and DBP of 1 mmHg taking the lower dose and 11 and 4 mmHg taking the higher dose.[42]
Milk Peptides
Compared with carbohydrate, dietary protein intake has been linked to reductions in SBP and DBP of 1.8 and 1.2 mmHg, respectively. This seems true for both vegetable and animal proteins.[43] Certain fish like sardines, tuna, mackerel, and bonito have been found to have ACE inhibition activity.[44]
Epidemiological studies have shown an association of lower BP with calcium (Ca) consumption.[45] This may be related to the Ca content in milk, as well as milk-derived proteins or peptides. It appears that when milk is fermented by specific bacterial strains (such as Lactobacillus helveticus), peptides are formed that have an inhibitory effect on the ACE system, leading to a blood-pressure-lowering effect.[46]
Pasteurization destroys the bacteria that activate these beneficial peptides. To obtain the blood-pressure-lowering effects of dairy, one would have to drink unpasteurized milk, which is not available in many areas, for various reasons. An alternative is to eat yogurt, which includes many of the bacteria that break down the milk proteins (e.g., casein and whey) into the beneficial peptides. Which types of yogurt contain the needed bacteria (L. helveticus) to create these ACE-inhibiting peptides is still being elucidated.
A 2016 trial (the Whey2Go Trial) found that consuming 56 gm/day of unhydrolyzed milk proteinswhey proteinfor 8 weeks lowered 24-hour ambulatory blood pressures (SBP down by 3.9, DBP by 2.5 mmHg), in addition to improving vascular reactivity and various laboratory findings.[47]
There are supplement companies marketing these peptides as a natural ACE inhibitor. There is limited benefit of using this type of remedy over ACE inhibitor medications, which are readily available.
Soy Protein
A double-blinded study showed that 40 gm of soy protein (the amount in one soy burger or two glasses of soy milk) lowered SBP by 7.9 mmHg and DBP by 5.3 mmHg in people with HTN.[48] Obtaining all protein from one food source is not ideal. There is some concern that high amounts of soy protein may increase the risk of bladder cancer, but protein from a variety of plant sources such as nuts, beans, and vegetables appears to be safe and beneficial.
Dark Chocolate
The cacao bean (Theobroma cacao) is rich in polyphenols such as procyanidins that have beneficial influences on arterial walls. A 2018 meta-analysis found that for the highest versus lowest category of chocolate consumption, the risk ratio of CVD dropped to 0.77.[49] A 2017 Cochrane review concluded that flavanol-rich chocolate and cocoa products cause a small (2 mmHg) BP-lowering effect in mainly healthy adults in the short term. There may be differential effects; that is, effects may be greater in people with prehypertension compared to people with lower pressures.[50]
Many brands of commercial chocolate are processed under conditions that destroy flavonoids, so look for gourmet chocolate that has at least 70% cocoa.[51] The daily dose: is 10-30 gm daily of dark chocolate (about 1/4 of a regular sized chocolate bar).
Garlic
A 2015 meta-analysis found that garlic had significant BP-lowering effects.[52] It brought SBPs down by 6.7 mmHg on average, and DBPs were reduced an average of 4.8 mmHg. A 2016 review found that for people with BP >140/90, reductions were even greater (SBP 8.7 and DBP 6.1 mmHg, on average).[53]
Beet Root (Beets)
Beet roots reduce BP through their effects on the nitrate/nitrite/nitric oxide pathways and can be considered a safe way to help control BP as part of an overall healthy diet.[54] Beets contain high levels of nitrate, and consuming them leads to dose-dependent effect of BP that can last for 24 hours.[55] After 4 weeks of consumption, mean reductions in BP were (for 24-hour ambulatory monitoring) a notable 7.7 mmHg SBP and 5.2 mmHg DBP.[36]
Coffee
A 2018 meta-analysis found that coffee consumption is inversely related to HTN, in a dose-response manner, noting that up to a point, risk of HTN dropped by 2% for each cup of coffee consumed daily. Some studies have questioned whether HTN risk might increase slightly if a person drinks more than 3 cups daily.[56] A review of green coffee extract supplementation (dose <400 mg) found benefits for SBP and DBP of 3.1 and 2.2 mmHg, respectively.[57]
Tea
Consuming 2-6 cups daily of either green or black tea over 1-6 months BPs, with more benefit when consumption was ongoing for longer than 12 weeks.[58] For green tea, the effect is higher (SBP, 2.1 and DBP 1.7 mm Hg) than for black tea (1.4 and 1.1 mm Hg).
Hibiscus tea, 2-4 cups daily for 2-8 weeks, also significantly reduces BP, including in people who are taking medications.[59]
Other Foods and Food Groups[17][60]
- Carbohydrates. Carbohydrates connection to BP are unclear, in terms of both how much of them are eaten and also the types of carbohydrates one eats.
- Copper and zinc. Copper and zinc levels do not seem to be independently associated with HTN.[61]
- Fats. Saturated fat does not affect BP, though it may have other effects on heart health. Monounsaturated fats modestly lower BP, but their benefits to heart health occur primarily through other means
- While fiber clearly has beneficial effects on lipids, it is not clear how much it directly effects BP. A 2005 study estimated that supplementing with 40-50 gm daily of a mixed fiber leads to a BP reduction of about 7.5/5.5 mm Hg.[62]
- As has been the case with the microbiome and so many physiological processes and illnesses, there is a connection between BP and the gut microbiome.[63] This is still being explored, but we know, for example, that various gut microorganism metabolites can alter gut function. Increased salt intake has a harmful effect on the microbiome. Probiotics may improve BP to a modest degree, particularly if it is elevated at baseline. One meta-analysis reported a change of 3.1 mmHg in SBP and 1.1 in DBP in people who drank a fermented milk product.[64]
- Vitamin C. The benefits of increasing vitamin C are unclear, according to some researchers, but one meta-analysis focusing on supplementing with 500 mg/day over 8 weeks found a SBP reduction of 4.8 mmHg, with no DBP effect.[65] People with low blood levels of vitamin C have a greater likelihood of being hypertensive.[66] Vitamin C may also make some drugs, like amlodipine, more effective.[67]
- Vitamin D. Vitamin D studies have somewhat complex findings. In general, BP effects seem to be limited. A meta-analysis found that effects depend on level of supplementation and population subgroup. Non-overweight patients, people over 50, and people taking >800 IU/day for <6 months seem to have the best results. People who take calcium supplements along with vitamin D actually show increases in BP in some studies[68], but calcium does seem to be beneficial for pregnant women because it reduces pre-eclampsia, pre-term birth, and overall complications.[69]
Moving the Body
Movement and physical activity benefit many aspects of health, beyond contributing to healthy BP levels. Whether patients are using exercise to lower BP, lose weight or improve aerobic capacity, appropriate exertion and persistence are keys to success. Tying in why activity matters to a person (its link to Mission, Aspiration, Purpose, or MAP MAP) is a key aspect of Whole Health care. It can take one to three months to see positive effects from increasing physical activity. If people do not exert themselves within safe parameters and do not maintain their activity levels over time, there may be little beneficial change.
A 2019 network meta-analysis of 391 trials compared the effect of exercise interventions to those of medications and concluded, Assuming equally reliable estimates, the SBP-lowering effect of exercise among hypertensive populations appears similar to that of commonly used antihypertensive medications.[70] A review of 16 studies concluded (in addition to benefits for many other risk factors) exercising over a period of >12 weeks reduced SBP by an average of 2.5 and DBP by an average of 2.3 mmHg.[20]
Physical fitness guidelines vary, but personalizing an activity plan for each patient can be a useful approach. More information can be found in the Moving the Body Whole Health overview.
In people with HTN, as with most adults, the recommendations are to complement aerobic exercise with muscle-strengthening activities that are moderate or high intensity and involve all major muscle groups on two or more days a week. It is clear in the research that endurance (aerobic), resistance, and isometric resistance training all lower systolic and diastolic BPs.[71] One trial focused on non-White adults found that dynamic resistance training has an equivalent or even greater benefit on BP compared to aerobic exercise.[72] A 2018 meta-analysis concluded that high-intensity interval training and moderate-intensity continuous training had similar beneficial effects.[73]
An easy tool to help understand how to obtain 50%-85% maximal oxygen uptake, which is recommended in many guidelines, is the perceived exertion scale, shown in Figure 1.
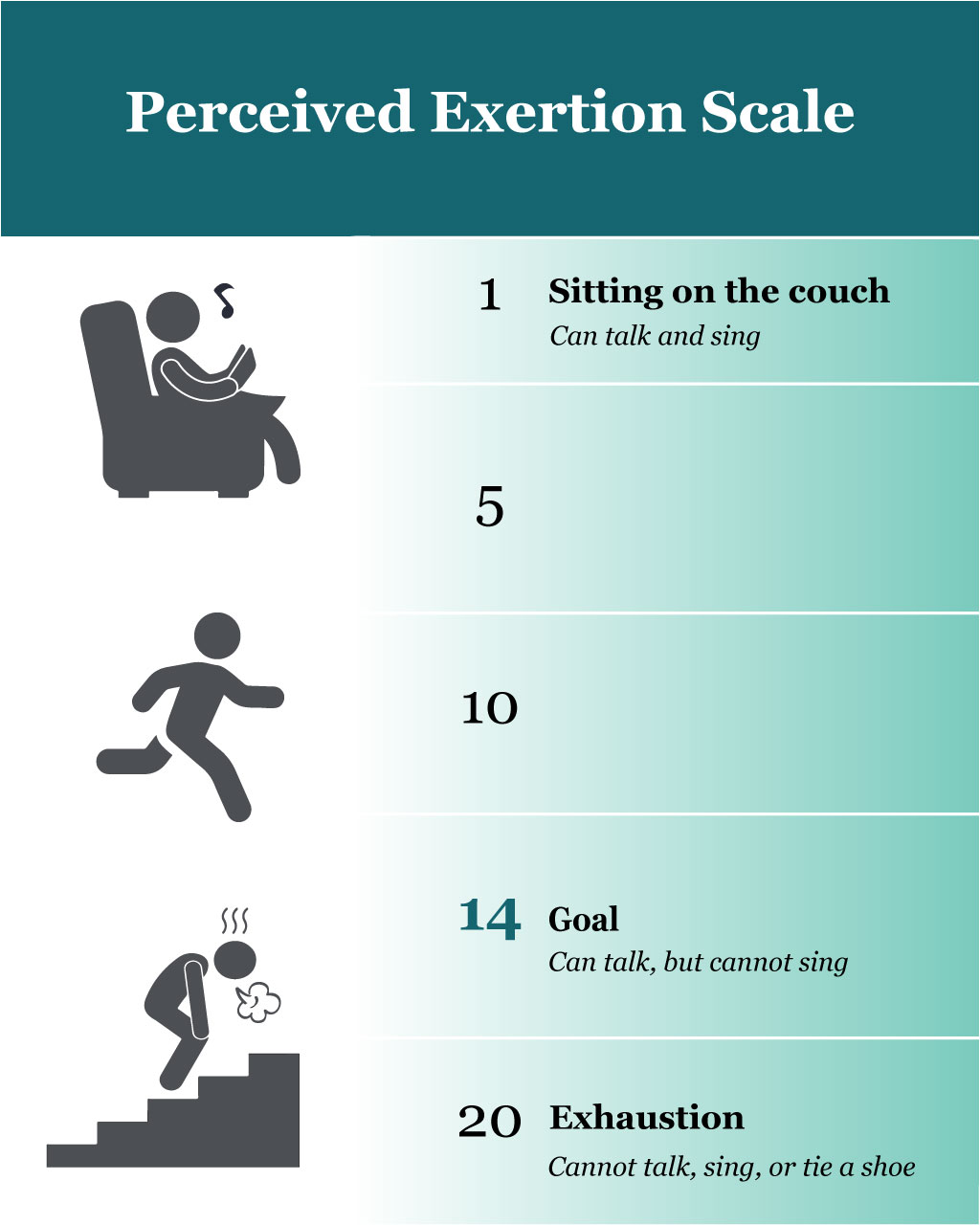
Perceived exertion. On a scale from 1-20, with 20 being the point of exhaustion, encourage patients to aim for 14 and work up to this as a maximum over a 25-minute period of time. At this level of exertion, a person should be able to talk but not sing. Exercise should occur on most days of the week. If a person does not have time for structured exercise each day, they can be encouraged to use stairs, walk briskly to lunch, or bike to work.
Yoga. A 2019 systematic review and meta-analysis in Mayo Clinic Proceedings concluded, based on data from 3,517 hypertensive adults, that practicing yoga an average of 5 sessions weekly for between 30-90 minutes per session, for 6-20 weeks (depending on the study) led to moderate reductions in SBP of 5 mm Hg and DBP of 3.9 mmHg.[75] Another review concluded that benefits overall were actually in the range of 10 mmHg for SBP and 8 mmHg DBP.[76] Of course, whatever the BP numbers indicate, yoga has other benefits as well. (The Yoga Whole Health tool has more details.)
Tai chi. Tai chi also seems to lower BP. A 2020 meta-analysis concluded that in a group of over 3,200 middle-aged adults, practicing an average of 4 times a week for 45-60 minutes, led to significant BP reductions.[77] Interestingly, studies in English found an average SBP drop of 10.4 mmHg, and DBP drop of 4 mmHg; Chinese studies reported decreases that were double those. The evidence for tai chi is reviewed in detail in Chapter 5 of the Passport to Whole Health.
Other specific activities, such as dance therapy, also show promise for reducing BP, but more research is needed.[78]
Recharge
Sleep quality has an effect on BP. Short sleep duration and poor sleep quality can both lead to substantial increases in BP. Normally, people should have a decrease in SBP and DBP of about 10%20% while sleeping. If the sympathetic nervous system remains active, this does not occur.[79] Obstructive sleep apnea (OSA) can lead to acute BP increases; people with moderate to severe OSA had 3.2 times the odds of developing hypertension.[80] The Recharge Whole Health overview has a number of suggestions about how to help a person improve their sleep.
Surroundings
BP increases based on external factors. There is an association between noise exposure and more clinically severe HTN symptoms.[81] People with fewer resources and those with established CVD seem more susceptible to the effects of environmental stressors on their BPs.[82] A 2017 review found that forest bathing (Shinrin-yoku) significantly reduces BP, while a person is in the forest environment.[83] It remains to be determined if there are long-term effects, or how this affects normotensive people versus those with HTN.
Personal Development
Education level is tied to better outcomes for people with HTN. A study of 223 patients with HTN found that education level was independently associated with risk.[84]
Spirit and Soul
A 2017 meta-analysis, while noting a great deal of variability among studies, concluded that psychosocial stress is associated with an increased risk of HTN (odds ratio of 2.40), and people with HTN experienced more stress compared to those with normal pressures (odds ratio 2.69).[87] Working with individuals around their specific stressors to individualize therapeutic approaches has the potential to increase their chances of success. Multiple factors can have an impact, ranging from urbanization, workplace stress, and lack of social connection.[88]
Emotions such as anxiety, hostility, and anger can influence the hypothalamic-pituitary axis, stimulating the sympathetic nervous system and resulting in the release of neurotransmitters and hormones that elevate BP. If the mind is constantly experiencing such emotions, the body may be bathed in these compounds with resultant elevations in BP and cardiovascular risk; being less emotionally reactive contributes to lower BP levels.[89] Conversely, emotional dampening (alexithymia) can also have negative BP effects.[90] As with so many aspects of our health, finding emotional balance is beneficial when it comes to BP.
Stress is as important a risk factor for development of CVD as truncal obesity and HTN. When a detailed list of risk factors were compiled from data coming from 52 countries, stress was only behind smoking in terms of factors that contribute most to CVD risk.[91]
In 20%-30% of people, stress increases susceptibility to the effects of salt on their BPs.[92] Low-stress resilience at age 18 was correlated with increased risk of HTN in a sample of over 93,000 men, with risk being higher for those who had a higher body mass index (BMI).[93] A 2018 study of stress management in the workplace found that employees with HTN did much better with their SBPs (but not DBPs) with an average drop of 7.5 mmHg when they used stress management techniques. Measures of depressive rumination and emotional exhaustion improved in parallel with reductions in BP.[94]
Mind-Body Tools to Lower Blood Pressure
There are a number of ways to reduce stress and thereby potentially prevent or treat HTN. Decisions about which mind-body tools to use must be based on individual preference, accessibility, and clinician experience about what might be the best fi for any given person. Less research is available for Guided Imagery and Clinical Hypnosis, as compared to meditation, breathing exercises, and biofeedback. A Cochrane review concluded there was insufficient evidence to guide practice around using Guided Imagery in pregnancy[95], and a 2016 meta-analysis reported that there is a trend toward BP benefit with music interventions.[96]
Meditation
In general, meditation seems to have significant favorable effects BP. A 2017 review of 19 studies found that SBP improved by 2.5 mmHg for transcendental meditation and an average of 3.8 mmHg for other types of meditation interventions; the latter did not meet statistical significance.[97]
Transcendental Meditation (TM), in which a person focuses on a word or mantra for 20 minutes a day for 16 weeks, was found to improve markers of the metabolic syndrome including HTN in individuals with known heart disease.[98] A 2017 review found that regular practice may reduce SBP by 4 and DBP by 2 mmHg, respectively.[99]
Mindful awareness meditation has shown similar benefits.[100] Mindfulness-based stress reduction (MBSR), which includes body-scanning and gentle yoga postures alongside other meditation techniques, resulted in BP being successfully lowered in the clinic setting, but it did not result in reduced ambulatory BP readings.[101][102] In people with cancer, those who took an MBSR course had decreased BP at 6- and 12-month follow-ups.[100] In a study of people with prehypertension, SBP, but not DBP, was significantly lowered (4.8 mmHg).[101]
Breathing Exercises
Taking deep breaths helps to balance autonomic nervous system activity and BP, while enhancing heart rate variability (HRV). Deep abdominal breathing stimulates the vagus nerve that travels through the diaphragm and stimulates the parasympathetic nervous system, leading to the relaxation response. When sympathetic tone is elevated, there is desensitization of the arterial and cardiopulmonary baroreceptors and BP can increase.
Slowing the breathing rate to less than 10 breaths/minute (ideal is 5-6 breaths/min) enhances baroreceptor sensitivity, lowering BP and increasing HRV. Slow breathing (8 breaths/minute) was found to lower baroreflex sensitivity in 60 people with HTN.[103] A stepwise-paced breathing procedure reduced SBP by an average of 8 mmHg, and DBP by an average of 5 mm Hg.[104] A 2019 meta-analysis of 17 studies found that device-guided slow breathing (breathing meets biofeedback) decreased SBP by 5.6 and DBP by 3 mmHg.[105] In older adults, slow-loaded breathing slow breathing accompanied by inspiratory muscle traininghelped reduce resting BP even for older people who had isolated systolic HTN.[106] A study of alternate-nostril yoga breathing also found reduced BP in the short-term.[107]
The Breathing Whole Health tool has additional information about breath-based techniques.
Biofeedback
For well over 20 years, studies have indicated that biofeedback-assisted relaxation improves BP, even several months after treatment.[108] A review of studies evaluating a biofeedback device designed to lower BP by modulating respiratory rate found that after eight weeks of treatment, there was an average drop of 14 mmHg SBP and 8 mmHg DBP.[109][110] The more the biofeedback device was used, the greater the drop. The recommended dose is 15 minutes of use each day with at least 45 minutes of slow breathing per week. Examples of biofeedback devices designed for personal use include the following (none of these is officially sponsored or endorsed by VA):
- RESPeRate (about $100). A device that uses sound and a respiration monitor one wears around the chest to slow breathing down. Goal is 5-6 breaths per minute. http://www.resperate.com/
- EmWave and EmWave2 Products (~$100 phone, $225 laptop or desktop version). Bluetooth model for smartphones is now available. http://www.heartmath.com/
- Unyte products (about $200 device cost, then $5/month; lifetime use $350). Biofeedback-guided meditation training software. https://unyte.com/collections/subscriptions.
- A number of other devices are also available online if one does a web search for stress management devices.
Similar results can be obtained using a simple breathing exercise. The key is to slow the breath to 6 breaths per minute, or one in and out breath every 10 seconds. Exhalation should be about twice as long as inhalation. Breathe in for a count of 3-4 and breathe out for a count of 6-7 and to take at least 60-80 intentional, slow breaths daily.
The Heart Rate Variability and Arrhythmias Whole Health tool has additional information about biofeedback and heart health.
Acupuncture
A 2018 Cochrane review looking at 22 trials with 1,744 people noted poor methodological quality because of high risk of bias due to lack of blinding, concluding that there was no evidence for a sustained BP lowering effect of acupuncture.[111] Low-quality evidence showed a short-term effect on pressures for 1-24 hours. Similarly, a 2018 meta-analysis found …there is inadequate high-quality evidence that acupuncture therapy is useful in treating HTN but did note that pooled results indicated that acupuncture plus medications had greater effects on BP than medications alone.[112] A 2019 review concurred that more studies are needed, finding that in 15 reviews, acupuncture was more effective in treating BPs than sham acupuncture, when both were used along with Western medicine.[113]
Dietary Supplements
Note: Please refer to the Passport to Whole Health, Chapter 15 on Dietary Supplements for more information about how to determine whether or not a specific supplement is appropriate for a given individual. Supplements are not regulated with the same degree of oversight as medications, and it is important that clinicians keep this in mind. Products vary greatly in terms of accuracy of labeling, presence of adulterants, and the legitimacy of claims made by the manufacturer.
A number of dietary supplements have been used to manage HTN. Most are noted in the VA formulary, but whether or not a clinician chooses to recommend them, it is important to have enough familiarity with them to guide patients and answer their questions about various products efficacy and safety. Note that beets, garlic, chocolate, green and black tea, hibiscus tea, and coffee are discussed in the Food and Drink section, above.
Most supplements used for HTN increase nitric oxide levels.[36] Someincluding cocoa, fish oil, and garlicalso increase angiotensin converting enzyme (ACE) inhibition. Olive oil, garlic, and olive leaf extract also seem to block calcium channels. Many other mechanisms of action have been posited as well.
Some important research findings related to dietary supplements and HTN include the following:
- Coenzyme Q10 (CoQ10). Also known as ubiquinone, CoQ10 is found in every cell in the human body and is used in the mitochondria to facilitate energy production. It works by an array of mechanisms, including through being a free radical scavenger, regenerating other vitamins and antioxidants, and supporting oxidative phosphorylation in the mitochondria. A 2009 Cochrane review found that treatment in people with pressures >140/90 led to decreases of SBP by an average of 11 and DBP 7 mmHg after four weeks of taking 100 mg daily.[114] However, a 2016 follow up Cochrane review found nonsignificant changes of 3.7 and 2.0 mmHg.[115] CoQ10 should be taken with a meal that includes some fat, to enhance absorption; it has a good safety profile. It seems to be beneficial when used with drugs that can deplete levels of CoQ10, such as statins, tricyclic antidepressants, and metformin.
- L-Arginine. Taken orally, the amino acid L-arginine, at doses ranging from 4-24 gm daily over 2-12 weeks, lowered SBP by 5.4 and DBP by 2.7 mmHg.[116] The beneficial effect is probably maximal after 4 weeks.
- Lycopene, a carotenoid found in red-toned foods like tomatoes, seems to have additive effects to medications for BP treatment.[117] SBP reduction was an average of 5.6 mmHg in people who took 10-50 mg/day for 4-12 weeks.[117]
- Long-acting forms of melatonin seem to improve sleep in people with HTN taking beta-blockers, and melatonin seems to cause a decrease in nighttime BPs (SBP 6.1 and DBP 3.5 mmHg.)
- Multivitamin, multimineral supplements (MVMMs). A large meta-analysis found that taking MVMMs might decrease SBP and DBP by 1.3 and 0.7 mmHg, respectively.[118]
- Pycnogenol, a bark extract from French maritime pine, allowed for a reduction by half of antihypertensive medications in one study.[119]
- Resveratrol is a polyphenol found in grapes. Doses of >150 mg daily of grape seed extract reduced SBP by 1.5 mmHg but did not significantly affect DBP.[120]
- Preliminary data suggest that black sesame, pomegranate juice, hawthorn, vitamin B6, alpha-lipoic acid, carnitines, and taurine may have slight but significant effects on BP. More trials are needed.[121]
In Conclusion
As you work with people to help them reduce their cardiac risk, blood pressure will necessarily be an important focus. Consider it within the larger picture of a persons Whole Health. Keep in mind the latest recommendations, tailor a plan to each individual patient, and stay aware of how all the different areas of self-care can help keep BP in range. Keep asking yourself what combination of medications, lifestyle changes, and other approaches will work best for any given person. Doing so has the power to lengthen and improve their lives.
Author(s)
Hypertension was written by Russell H. Greenfield, MD and updated by J. Adam Rindfleisch, MPhil, MD (2014, updated 2020). Sections were adapted from Non-Pharmaceutical Therapy for Hypertension by David Rakel, MD.



















