Generic Procedures
Generic procedure templates for EM labs will be posted on this page for use as a guide in developing site specific EM procedure manuals. If you have any procedures that you feel would be beneficial to share with other EM staff, please send them to: Edoris.LeFurgey@va.gov
The following generic EM biopsy processing procedures are now available for use as possible specimen processing procedure templates. Click on following links to view the section on: Kidney bx, Nerve bx, Tissue bx. To download the procedures in Microsoft Word document format, click on kidney.doc, tissue.doc or nerve.doc.
SUMMARY OF PROCEDURE FOR KIDNEY BIOPSIES
The kidney biopsy (needle or open wedge) requires special handling. Materials and reagents required include (1) a specimen container with buffered formalin, (2) a specimen container with 3% glutaraldehyde in 0.1M sodium cacodylate buffer, (3) a specimen container with immunopathology fixative (Michel's fixative; Zeus medium), (4) a bottle of phosphate buffered saline (PBS), (5) a specimen container with a 2 X 2 gauze, (6) a pair of forceps, (7) a single edge razor blade, (8) a sheet of wax, and (9) a VA Surgical Pathology Request Form (SF 515).
The biopsy should first be placed on the 2 X 2 gauze previously moistened with PBS to prevent desiccation, placed in a clean, empty specimen container, and transported as rapidly as possible to the Surgical Pathology Laboratory.
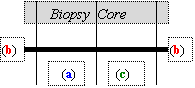
Once in the laboratory, the biopsy should immediately be divided and and fixed as follows: Place the cores on the sheet of wax in several droplets of PBS; be careful to keep PBS on the biopsies at all times. Place under a dissecting microscope and divide according to the scheme illustrated.
|
|||||||||||
a. Tissue for Immunofluorescence/Immunoperoxidase Studies
A portion of the central region of the core(s), from the cortex and ideally containing one or more glomeruli, should be excised for immunostaining. The specimen should be placed in the immunopathology (Michel's) fixative and kept at room temperature. Care must be exercised at this point to avoid contamination of the specimen with formalin or glutaraldehyde, as this precludes successful immunofluorescence or immunoperoxidase analysis.
b. Tissue for Electron Microscopy
One to four very small (1 mm) sections of renal cortex, ideally also containing glomeruli, should be cut from the ends of the biopsy core and placed in the bottle of glutaraldehyde for ultrastructural studies. Buffered formalin is an acceptable although not ideal alternative to glutaraldehyde for fixation and shipping.
c. Tissue for Routine Surgical Pathology
The remaining tissue should be placed in the specimen container with formalin for conventional paraffin embedding and histological examination by light microscopy.
If a limited amount of tissue is available, priority should be given to the portion allocated for routine surgical pathology.
Label all containers with the patient's name and social security number. On the accompanying SF515, include information regarding the type of biopsy (medical, transplant, or transplant donor; needle or wedge), pertinent clinical history (including current medications), and who should be contacted with results (including phone and/or Pager ID numbers).
Make sure all caps on vials are secure and seal with parafilm. Transport or mail all specimens to:
-
Surgical Pathology Laboratory, Room XXXX
-
Pathology and Laboratory Medicine Service, 113
-
Local VA Medical Center
-
Street
-
City, State Zip
-
Be sure to label package on outside as “URGENT: PATIENT SPECIMENS." Specimens obtained after hours or on weekends should be held in the appropriate fixatives and mailed on the next work day.
REAGENTS FOR KIDNEY BIOPSIES
1. Electron Microscopy
Buffer
0.2 M Sodium Cacodylate Buffer
-
42.8 gm. Na cacodylate
-
fill to 1000 ml. with distilled H2O
-
Adjust pH to 7.4 using 37% HCl
-
Osmolality 262 mOsm.
-
Store in refrigerator for one year.
Fixative
-
Glutaraldehyde comes in 25% sealed glass ampoules. Keep refrigerated.
Amount of stock glut. to use = (% desired x volume desired) / % stock
Half of the final volume should be 0.2 M Na Cac. buffer so final concentration is 0.1 M.
For 100 ml of 3% made from 25% stock:
(3 x 100 ml) / 25 = 12 ml.
-
to 12 ml. 25% glutaraldehyde
-
add 50 ml. 0.2 M Na cacodylate buffer
-
and 38 ml. distilled H2O
-
pH should be 7.2
-
osmolality 600-650 mOsm.
Store in refrigerator for one month.
Label with name of solution and concentration, pH, osmolality, preparation date, expiration date and initials of person preparing solution.
2. Immunofluorescence
-
Michel’s Fixative--available commercially from Wampole/Carter Wallace, P.O. Box 1001, Cranbury, NJ 00512; 800-257-9525; Vendor Stock # 0102; 10 ml/bottle, 12 bottles/case; purchase by the case; Michel’s Washing Solution, Vendor Stock# 0103.
3. Light Microscopy (Conventional Histology )
-
10% Formalin is available commercially from Fisher Scientific or others
Generic template -
SUMMARY OF GENERAL PROCEDURES FOR TISSUES
TYPES OF TISSUE
1. Biopsy or autopsy tissue; usually tumors.
2. Kidney, nerve, muscle, buffy coat, etc: please refer to specific sections for details concerning the proper collection of these tissues. Contact one of the EM staff individuals listed at the bottom for additional details as required.
COLLECTION OF TISSUE
NOTE: A specimen should also be obtained for routine light microscopic evaluation at the time the specimen is obtained for EM Study. EM is not a substitute for routine light microscopy. When renal biopsies are obtained, immunofluorescence studies should also be considered.
1. Tissue should be placed in buffered glutaraldehyde as soon as possible; for fresh tissue, within 2 minutes of the biopsy. (Local VAMC) EM Laboratory will send buffered glutaraldehyde (3% glutaraldehyde in 0.1M sodium cacodylate) to other laboratories on request.
2. Place several drops of buffered glutaraldehyde onto a sheet of wax. Using a fresh razor blade, cut the tissue into very small (1 mm) cubes. Transfer about 10-20 such pieces to a small bottle (e.g. glass scintillation vial) containing a large volume of fixative (about 10 ml). The minimum amount of tissue is a single grossly visible (~0.5 x 0.5 x 0.5 mm) fleck of appropriately selected tissue. The ideal temperature of the fixative is room temperature. Tissue can be stored/shipped at room temperature. Buffered formalin is an acceptable although not ideal alternative to glutaraldehyde for fixation and shipping.
COLLECTION OF SERUM, SPINAL FLUID, STOOL, URINE, OR OTHER FLUIDS FOR EM EXAM OF VIRUSES – Contact the EM lab for specific instructions, but generally specimens diluted with media, buffer or fixative are not acceptable; Refrigerate “pure” specimen after collection.
PACKAGING AND SHIPPING
Label all containers with the patient's name and social security number. On the accompanying SF515, include information regarding the type of tissue, pertinent clinical history (including preoperative diagnosis if applicable), and who should be contacted
Please, also include a copy of your surgical pathology report (if available) and/ or comments concerning the questions to be addressed by electron microscopy. It is helpful if a representative H&E slide can be sent as well. (This slide will be returned.)
Make sure all caps on vials are secure and seal with parafilm. Transport or mail all specimens to:
Electron Microscopy Laboratory, Room XXXX
Pathology and Laboratory Medicine Service, 113
Local VA Medical Center
000 Local Street
City, State Zip
Be sure to label package on outside as “URGENT: PATIENT SPECIMENS”. Specimens obtained after hours or on weekends should be held in the appropriate fixatives and mailed on the next work day.
Address questions concerning submission of tissues or other materials for electron microscopy to Local Names/contacts/addresses/phone numbers.
Generic template -
SUMMARY OF PROCEDURES FOR NERVE BIOPSIES
OPERATING ROOM PROTOCOL
Be certain that Glutaraldehyde containers are clearly labeled "GLUTARALDEHYDE" (as a minimum). If a clinician in the Operating Room mistakes Glutaraldehyde for Saline, the result may be a medical disaster!
Supplies/Reagents/Equipment Required in Operating Suite at Initiation of Procedure:
¨ 3% Glutaraldehyde in 0.1M sodium cacodylate buffer
¨ Muscle/Nerve Biopsy Clamps (Rayport Muscle Biopsy Clamp # SU 130-1112, Allegiance Healthcare Corp., Government Customer Service, 3651 Birchwood Drive, Waukegan, Illinois 60085, Telephone 800-444-1166) or Wooden Applicator Sticks
¨ Zeus/Michel’s Fixative for Immunofluorescence (Wampole Labs, Division of Carter-Wallace, PO Box 1001, Cranbury, NJ, Telephone 800-257-9525, Ext 3; Catalog # 0102)
¨ Zeus/Michel’s Washing Solution, see above, Catalog# 0103
Clamped Specimens for Light Microscopy and Electron Microscopy
¨ Expose nerve (generally sural nerve) by gentle dissection, with care taken not to traumatize the nerve
¨ Clamp the nerve (or a single nerve fascicle) in situ with muscle/nerve biopsy clamp or suture the nerve in situ to a wooden applicator stick
¨ Section nerve free at either end of clamp/stick and remove with nerve attached to clamp/stick
Place IMMEDIATELY into 3% Glutaraldehyde in buffer.
Note: Fixation in glutaraldehyde after removal must be as rapid as possible
Glutaraldehyde fixation is continued overnight (or longer, if a weekend) in the refrigerator until the specimen in glutaraldehyde is packaged for shipping as described below. The specimen in glutaraldehyde must remain on the muscle clamp for at least 4 hours. At the end of the 4 hours, the tissue may be unclamped, but it still must remain in glutaraldehyde until further processing occurs. Label all containers with the patient's name, social security number, type of specimen and type of fixative in the container.
Unclamped Specimens for Immunofluorescence
Note: Fixation in glutaraldehyde after removal must be as rapid as possible
Glutaraldehyde fixation is continued overnight (or longer, if a weekend) in the refrigerator until the specimen in glutaraldehyde is packaged for shipping as described below. The specimen in glutaraldehyde must remain on the muscle clamp for at least 4 hours. At the end of the 4 hours, the tissue may be unclamped, but it still must remain in glutaraldehyde until further processing occurs. Label all containers with the patient's name, social security number, type of specimen and type of fixative in the container.
Unclamped Specimens for Immunofluorescence
electron microscopy reports (if available) and/ or comments concerning the questions to be addressed by electron microscopy. It is helpful if a representative H&E slide can be sent as well. (This slide will be returned.)
Make sure all caps on vials are secure and seal with parafilm. Transport or mail all specimens to:
Electron Microscopy Laboratory, Room XXXX
Pathology and Laboratory Medicine Service, 113
Local VA Medical Center
000 Local Street
City, State Zip
Be sure to label package on outside as “URGENT: PATIENT SPECIMENS”. Specimens obtained after hours or on weekends should be held in the appropriate fixatives and mailed on the next work day.
Address questions concerning submission of tissues or other materials for electron microscopy to: Local Names/contacts/addresses/phone numbers
NOTES:
1. The nerve tissue fixed in glutaraldehyde as described above will be studied by light and electron microscopy. If indicated, an aliquot of this same sample can also be studied by nerve fiber teasing as well.
2. Please note that the above protocol does not use formalin for any portion of the nerve biopsy. Rapid fixation in glutaraldehyde is the preferred method of preservation. Excess tissue can be embedded in paraffin for routine histology.
SUMMARY OF PROCEDURES PERFORMED FOR NERVE BIOPSIES ON SITE AT Local VAMC
VAMC ELECTRON MICROSCOPY PROTOCOL
The nerve specimen is removed from the clamp, and rinsed in buffer. After the segments of nerve crushed by the clamp have been cut away and discarded, a single cross section is taken from each end, post-fixed in osmium and embedded in epon. Cross-sections, 1 mm in thickness, are stained with toluidine blue for light microscopy. In selected cases thin sections will be cut for electron microscopy. The remaining portion of nerve may be dehydrated through graded alcohols into cedar wood oil for teased nerve preparation if indicated. (Individual myelinated axons are placed on a clean glass slide, partially dried, and coverslipped with permanent mounting medium.)
VAMC IMMUNOFLUORESCENCE PROTOCOL
Specimens are removed from Michel’s fixative and washed in buffer (Michel/Zeus Washing Solution, *see reagents). Excess buffer is removed with a blotter. The specimens are embedded in OCT medium with tissue cores parallel to the eventual plane of sectioning, then snap frozen in a cryostat with a heat sink or in liquid perfluohexane (-90C). Frozen sections are cut at 3 microns on a cryostat and collected on coated slides (positively charged, e.g. Superfrost Plus™). The sections are stained by the direct immunofluorescence method using FITC-conjugated antibodies and coverslipped in aqueous mounting medium. The sections are viewed by a pathologist using an epifluorescence microscope possessing a mercury light source. The light is passed through an excitation filter, yielding a wavelength range of 470-510 nm, which causes the FITC label to fluoresce.