Gastroesophageal reflux disease (GERD) is an extremely common condition, affecting nearly 1 in 5 U.S. adults at least weekly and nearly 1 in 10 daily.[1][2][3] It affects women more commonly than men, and the peak ages are 30 to 60.[4]
Symptoms of GERD occur due to esophageal irritation from acidic stomach contents (including pepsin and sometimes bile acids) contacting the esophagus through the lower esophageal sphincter (LES).
A variety of symptoms can occur:
- Retrosternal discomfort (e.g., pain, burning)
- Acid regurgitation
- Nausea and/or vomiting
- Laryngitis
- Cough
- Dental erosions
- Wheezing
- Difficulty swallowing
Normally, the LES only relaxes when one is swallowing food. Otherwise, it has enough tone to limit retrograde flow of acidic contents into the esophagus. With long-term acid exposure, the esophagus may become inflamed (esophagitis) and constrict (stricture), and it can also develop columnar metaplasia (Barretts esophagus) or adenocarcinoma.
There are many factors that cause GERD, and these should be systematically evaluated when creating a treatment plan that aims to cure rather than just treat this disease. The quality-of-life burden is significant and may be greater than that of congestive heart failure, coronary heart disease, and diabetes.[5]
Although the LES itself may appear to be the site of dysfunction, it may not play the primary role in GERD pathogenesis. The problem may be downstream, due to increased intra-abdominal pressure (e.g., from obesity, pregnancy, restrictive clothing, ascites). It may also be caused by poor GI motility (gastroparesis) or from forces that challenge normal forward motility (recumbent position, bending over). It may also be due to compromised esophageal mucosal barrier (e.g., from low saliva production). This etiology is often overlooked; one study showed that when acid is infused directly into the esophagus, 88% of those with GERD were symptomatic, whereas only 15% of controls without known GERD had symptoms.[6] Further, the significant overlap between GERD and dyspepsia (25% of those with GERD have both[7]) likely supports the fact that these two entities may not be discreet diagnoses but may have shared underlying mechanisms.
Studies using proton pump inhibitors (PPIs) and histamine-2 (H2) antagonists show significant benefits in their placebo groups. This supports the notion that the mind has significant potential to affect GERD symptoms. In a large meta-analysis, placebo rates averaged about 20% for pharmaceuticals, indicating that any GERD intervention that one expects benefit will provide a positive response for 1 in 5 people independent of the mechanism of action. Creating this positive expectation through a therapeutic clinical relationship can therefore be an important aspect of developing a Personal Health Plan (PHP).
Role of Prevention and Screening
Primary prevention of GERD should be based on health screening and prevention recommendations that apply to all Veterans. Perhaps the most important of these include maintaining a healthy weight, avoiding tobacco products, and limiting excess alcohol consumption. Although there is inconsistent evidence on which foods may provoke symptoms (food eliminations must be individualized), it is clear that avoiding large meals and eating within 2-3 hours of bedtime can improve symptoms.[8] In those with a previous personal history or family history of GERD, it may also be prudent to avoid potentially provocative medications, when possible. These include:
- Aminophylline
- Anticholinergics
- Beta-adrenergics
- Calcium channel blockers
- Nitrates
- Phosphodiesterase inhibitors including sildenafil
Dietary Supplements
Note: Please refer to the Passport to Whole Health, Chapter 15 on Dietary Supplements for more information about how to determine whether or not a specific supplement is appropriate for a given individual. Supplements are not regulated with the same degree of oversight as medications, and it is important that clinicians keep this in mind. Products vary greatly in terms of accuracy of labeling, presence of adulterants, and the legitimacy of claims made by the manufacturer.
Melatonin
Dose: 3-6 mg, 30-90 minutes before bedtime. One of melatonins physiologic functions is to increase the LES tone. Endogenous melatonin peaks in the evening, thus it may be one way that innate physiology can help to minimize gravity-dependent reflux. Supplementing with melatonin may not only help with sleep initiation, but it may be as effective as a low dose PPI in treating GERD.[8][9]
Deglycyrrhizinated licorice (Glycyrrhiza glabra)
Dose: 2-4 380 milligrams lozenges before meals.[10] This botanical medicine, like several others on this list, is a demulcent (or mucilaginous). It enhances esophageal mucosal protection. The deglycyrrhizinated form of licorice (DGL), as the name implies, does not contain glycyrrhizin, which has mineralocorticoid actions, such as hypertension, hypokalemia, and edema.[11] Do not use tablets. Rather, use the lozenges, as they can be chewed and swallowed slowly to allow effective contact with the lower esophagus.
Slippery elm (Ulmus fulva)
Dose: 1-2 tbsp powder mixed with 1 cup water after meals and before bedtime. Slippery Elm is also a demulcent that is useful for GERD. The root bark powder needs to be carefully titrated with water to ensure a palatable consistency. To enhance flavor, consider adding a small amount of honey or maple syrup. This herb has an excellent safety profile, though it has the potential to bind to certain medications and decrease their absorption.[11]
Marshmallow (Althea officinalis)
Dose: 2-3 tsp, in divided doses, as an infusion of leaves or root.[12] This is another mucilaginous herb with similar properties to slippery elm. Again, this may inhibit the absorption of some medications.[11]
Chamomile (Matricaria recutita)
Dose: 1 tbsp dried flowers per cup hot water as a tea 3-4 times daily.[13] This botanical has antispasmodic effects on the GI tract, but its use for GERD likely comes from its anti-inflammatory effects.[11] Be mindful if one has allergies to plants in the daisy family (Asteraceae), as there may be some cross-reactivity.
Other Systems
Traditional Chinese medicine (TCM) provides a comprehensive diagnostic and therapeutic framework for treating individuals with diseases such as GERD through lifestyle changes, botanical medicines, and other modalities, such as acupuncture.
Acupuncture may have clinical efficacy for GERD based on three possible mechanisms. Acupuncture may stimulate GI motility and decrease acid secretion via the vagus nerve and other parasympathetic pathways. Acupuncture may also increase esophageal sensory thresholds, which decrease in those with GERD. It also found that the combination of acupuncture plus pharmaceuticals is more effective than either one alone.[14] Acupuncture may also be beneficial for treating functional dyspepsia.[8]
Acupuncture may also be more effective than doubling the dose of a PPI in those with persistent symptoms on standard-dose PPIs.[15]
Consider acupuncture based on ones preferences, if it is easily and widely available, and/or if Western/Eastern medicine treatments have proved ineffective. It may also be a valuable tool in helping one to wean off a PPI (refer to the section below).
A Note About Chronic PPI Use
Although pharmaceuticals can be very effective in relieving GERD symptoms, there are increasing concerns of the harmful effects of chronic acid suppression. In our attempts to balance risks and benefits, and to first do no harm, the following observations should be routinely considered when deciding whether to continue chronic acid suppression therapies.
Digestion
Incomplete digestion of protein. Proteins begin to be digested in our stomach directly by hydrochloric acid and indirectly though hydrochloric acids activation of pepsin. Pepsin levels fall within a few days of starting a PPI. One hypothesis that may explain increasing rates of eosinophilic esophagitis is that the esophageal immune system is reacting to more incompletely digested proteins due to decreased activation of pepsin.[16][17]
Decreased absorption of B12,[18][19] iron (especially nonheme),[20][21] and calcium.[22] Hydrochloric acid often plays a role in removing vitamins from their carrier proteins, making these accessible for assimilation into our body.
Immunity
Increases in community-acquired Clostridium difficile infection. In one large case-control study, use of PPI and H2-antagonists at least doubled rates of this infection (4% absolute increase with H2-antagonist, 15% absolute increase with PPI).[23]
Increases in community-acquired pneumonia. In adults, a large cohort study showed one increased case in 226 of those treated with PPIs and 508 of those treated with H2-antagonists.[24] In children, the rate of pneumonia is 10 times higher for those on these medications.[25]
Increases in acute gastroenteritis in children. Many children are now being treated for reflux-type symptoms. After 2 months of use in young children aged 4-36 months, their risk of developing gastroenteritis more than doubles.[25]
Cancer
Enterochromaffin cell hyperplasia. Chronic acid suppression causes an adaptive increased secretion of gastrin as the body attempts to restore normal gastric acidity. This leads to a hypergastrinemic state that has been shown to be a risk factor for tumor development.[26]
Increased gastric carcinogens. Lower gastric acidity equates to increased gastric bacterial load. One hypothesis is that this environment may lead to increased conversion of dietary nitrates to nitrites. The nitrites are then converted to the carcinogenic compound N-nitrosamine.
Fractures
Associations with long-term acid suppression have been found for hip[27][28] and spine[29][30] fractures. This may be related to impaired calcium absorption, as described above.
Many clinicians place people with GERD on medications with no real thought given to underlying causes, which end up never being addressed. Some patients may try to discontinue PPI therapy on their own, only to have a sudden return of symptoms. However, this return of symptoms may be predictable and not necessarily indicative of continuing GERD pathology. When healthy and asymptomatic people are given 40 mg of pantoprazole for 6 weeks, they will get 10-14 days of GERD symptoms when they stop therapy.
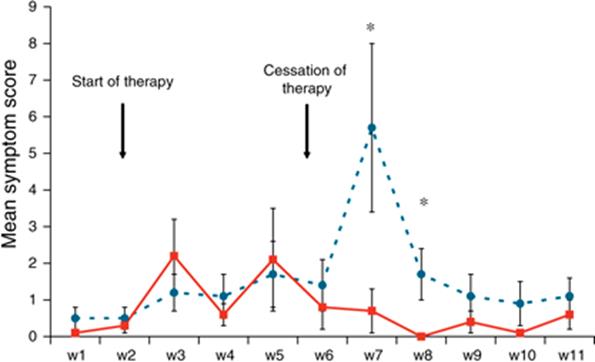
Tapering Off a PPI
After someone has made appropriate lifestyle changes, and after underlying causes have been addressed, it may be appropriate to a taper off his or her PPI. Rather than simply decreasing the dose over 2-4 weeks, a combination of modalities should be added to maximize ones chances for success. For more information, refer to Coming Off a Proton Pump Inhibitor Whole Health tool.
Summary of Nonpharmaceutical Options for GERD
Based on the Strength of Recommendation Taxonomy (SORT) criteria, there are no known nonpharmaceutical therapies for GERD with consistent, good-quality, and patient-oriented evidence. To receive an A rating, a therapy needs to be supported by a systematic review or meta-analysis showing benefit, a Cochrane review with clear recommendation, or a high-quality, patient-oriented randomized controlled trial. The following therapies are based on inconsistent or limited-quality, patient-oriented evidence and would receive a B rating:
- Nearly daily moderate to vigorous exercise for 30-60 minutes, away from mealtime
- Food elimination diet
- Increase fiber to meet general recommendations
- Elevate head of bed (consider only in those with severe symptoms)
- Avoid large meals and eating 2-3 hours before bedtime
- Acupuncture
- Melatonin
- Diaphragmatic Breathing
The following therapies, based on consensus, usual practice, opinion, disease-oriented evidence or case series, would receive a C rating:
- Botanicals (licorice/DGL, slippery elm, marshmallow, chamomile)
- Relaxation techniques (e.g., meditation, biofeedback, progressive muscle relaxation, journaling)
Author(s)
Gastroesophageal Reflux Disease was written by David Lessens, MD, MPH (2014, updated 2020). Sections were adapted from Gastroesophageal Reflux Disease by David Kiefer, MD, David Rakel, MD, and Rian Podein, MD.



















