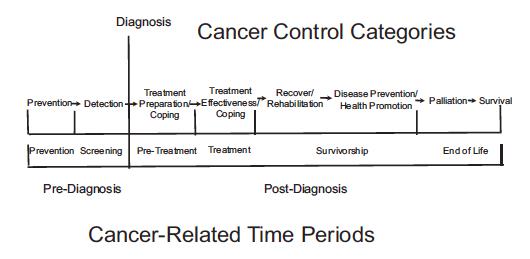
Whole Health emphasizes mindful awareness and Veteran self-care along with conventional and integrative approaches to health and well-being. The Circle of Health is a key component of Whole Health that highlights eight areas of self-care: Surroundings; Personal Development; Food & Drink; Recharge; Family Friends, & Co-Workers; Spirit & Soul; Power of the Mind; and Moving the Body. The narrative below models a Whole Health clinical visit by applying the latest research on various self-care approaches and complementary and integrative health (CIH) to cancer care.
A Whole Health approach to cancer care incorporates nutrition, dietary supplements, yoga, meditation, acupuncture, and other integrative and complementary practices to empower patients and their families.
Meet the Veteran
 Pam is a 48-year-old nurse who served in Iraq during Desert Storm. She recently found a painless 1 cm lump in her left breast that grew over the past two months. Her VA clinic physician ordered a mammogram which showed a concerning lesion. The biopsy revealed estrogen and progesterone negative breast cancer. After a mastectomy and axillary node dissection, it was confirmed that the cancer had metastasized to some of the local lymph nodes. Her oncologist explained that her cancer treatment will need to include chemotherapy and radiation.
Pam is a 48-year-old nurse who served in Iraq during Desert Storm. She recently found a painless 1 cm lump in her left breast that grew over the past two months. Her VA clinic physician ordered a mammogram which showed a concerning lesion. The biopsy revealed estrogen and progesterone negative breast cancer. After a mastectomy and axillary node dissection, it was confirmed that the cancer had metastasized to some of the local lymph nodes. Her oncologist explained that her cancer treatment will need to include chemotherapy and radiation.
Pam is devastated by the news. She has many responsibilities as a single mother of two teenage sons working a full-time job. She is a former smoker with a 25-pack/year history, and the stress of the cancer diagnosis has caused her to pick up smoking again to calm her nerves. In addition, she is having trouble sleeping, which is making her fatigued at work. She is worried that the anxiety will get in the way of providing good nursing care to her patients. Her life stress over the past 10 years has contributed to a 30-pound weight gain, making her overweight.
Pam is also worried about the short- and long-term side effects of treatment. She hates that she will end up losing her long hair and will need a breast implant. Who will take her to and from treatments? Will the chemotherapy leave her too weak to continue working full-time? Who will watch the children during chemo? She is seeking comprehensive care that will include things that will support conventional therapy while reducing side effects. She also wants help making lifestyle changes to reduce the chance of breast cancer recurrence.
Personal Health Inventory
On her Personal Health Inventory (PHI), Pam rates herself a 2 out of 5 for her overall physical well-being and a 1 for overall mental and emotional well-being. When asked what matters most to her and why she wants to be healthy, Pam responds:
“I love spending time with my children. I want to be healthy enough to watch my sons grow up and support them in every way possible. I also want to be a great nurse and touch as many lives as I can.”
For the eight areas of self-care, Pam rates herself on where she is and where she would like to be. She decides to first focus on the areas of Moving the Body and Food and Drink by making time for jogging and preparing meals at home.
For more information, refer to Pam’s PHI.
Introduction
Registry data in 2007 indicate that over 97% of VA cancers were diagnosed in men, with cancer type incidence mirroring that of U.S. males.[1] The five most common cancers diagnosed were prostate, pulmonary, colorectal, bladder, and melanoma.[1] Of course, female Veterans like Pam are also affected.
More than half of cancer patients in the United States use complementary approaches during the survivorship period following acute cancer therapies,[2] but up to 60% do not inform their health care team.[3] Patients often use complementary therapies because they want to optimize their chances of survival. However, this can challenge and frustrate physicians who have limited knowledge of complementary medicine, thereby discouraging patients to disclose their use.
It is critical to inquire about the use of complementary approaches as a routine part of initial evaluations of cancer patients and to decide together on therapeutic management options at each stage of cancer care. The dangers of not engaging patients in an open discussion of integrative treatments include patients holding false beliefs in a “cure-all” therapy, interactions of supplements and botanicals with chemotherapy and radiation, and patient mistrust of conventional therapies reducing compliance to treatment.
Patients diagnosed with cancer face a different internalization process than those diagnosed with a noncancerous chronic medical condition. It is compounded by periods of extreme fear worsened by the uncertainty of recovery and a heightened awareness of their mortality as the disease progresses. Those with cancer often describe the experience as a journey, which is unique for each individual. Even if the cancer is cured, patients will find themselves adjusting to a “new normal” while they become accustomed to residual side effects from treatment and learning what new symptoms may or may not be concerning for a recurrence.
Important elements in a Whole Health approach to cancer care[4]
- Communicate empathically with compassion and care.
- Provide adequate information and knowledge from reliable sources about complementary approaches.
- Provide psychological, social, and spiritual support.
- Empower patients and their families.
- Enable patients to develop strategies for living with cancer and support them in the process.
- To help cancer patients be truly informed and autonomous, clinicians need to do the following:
- Learn about conventional treatments that they have tried, failed, or rejected because of safety, quality of life, cost, or other issues.
- Discover the levels of support that the patient relies on from family, community, faith, and friends.
- Ask patients about their use of complementary approaches or interest in using complementary medicine.
- Identify the patient’s beliefs, fears, hopes, expectations, and experience with complementary approaches.
- Acknowledge the patient’s spiritual and religious values and beliefs, including views about quality of life and end of life, and seek to understand how these issues affect health care choices.[5]
Mindful Awareness
Mindful awareness is an overall approach that has relevance for people with many different diagnoses. There are a few studies where its use has been found to benefit people with cancer. A 2013 study of 268 individuals with cancer found that mindful awareness training significantly reduced mood disturbance and stress. Present moment awareness and avoiding judging inner experiences were the two skills most closely linked to improvements in psychological functioning.[7] A 2004 study of 59 patients with breast cancer and 10 with prostate cancer found that mindful awareness training (in the form of a mindfulness-based stress reduction course) was associated with enhanced quality of life and decreased symptoms in both groups.[8] For more information about the effects of structured mind-body approaches on people with cancer, refer to the Power of Mind section below, as well as the “Mindful Awareness” Whole Health overview.
Self-Care
Moving the Body
Exercise
Physical activity and nutrition counseling have not traditionally been part of cancer treatment and survivorship programs. The American Cancer Society (ACS) cites evidence that regular physical activity may reduce the risk for breast, colon, endometrial, and advanced prostate cancer, and possibly pancreatic cancer. The ACS recommends at least 150 minutes of moderate intensity activity or 75 minutes of vigorous-intensity activity a week to see a protective benefit.[9] Cancer risk reduction with physical activity ranges from 10%-30%, with best evidence for colon and breast cancer and moderate evidence for endometrial, prostate, and lung cancer.[10][11]
Four large observational studies have demonstrated that engaging in regular moderate-intensity physical activity after the diagnosis of breast cancer is associated with a 24%-67% reduction in the risk of total deaths and a 50%-53% reduction in the risk of breast cancer deaths compared with women reporting no exercise.[12][13][14][15] The maximal exercise benefit occurred in those who performed the equivalent of brisk walking 3 hours per week. Two large observational studies have demonstrated that the same amount of physical activity after the diagnosis of colon cancer is associated with a 50%-63% reduction in the risk of total death and a 39%-59% reduction in the risk of colon cancer death relative to people who do not exercise at all.[16][17]
A prospective cohort study of patients with metastatic lung cancer found that functional capacity, as measured by a 6-minute walk test, was an independent predictor of overall survival. In addition, patients reporting greater than 9 MET-hours of exercise per week had prolonged survival compared with patients who reported less, 26 versus 13 months, respectively.[18] (Note: MET-hours is a measure of exercise intensity. It stands for “metabolic equivalents.” This measurement system allows for discussion of the relative intensity of different types of exercise. People burn 1 MET per hour if they are sedentary. Twenty-three METs is the intensity of running close to a 4-minute mile.) A similar prospective cohort study of patients with recurrent malignant glioma found that greater than 9 MET-hours per week of exercise was associated with a median survival of 22 months, whereas less was associated with a median survival of 13 months.[19]
Cardiorespiratory fitness measured by maximal oxygen uptake (VO2max) is an important predictor of all-cause mortality. Lower VO2max values have been observed in patients with cancer compared to healthy individuals. Longer weekly exercise durations (150 minutes) and larger weekly exercise volumes result in larger beneficial changes of VO2max in patients with cancer who are undergoing treatment.[20] For patients receiving treatment for breast cancer, physical performance is better when using a supervised vs. unsupervised training program, and incorporating resistance-training decreases perceived fatigue.[21]
Exercise also has positive effects on many factors contributing to cancer-related fatigue including muscle strength, cardiopulmonary fitness, aerobic capacity, sleep quality, pain, and mood disturbances, with benefits during or after cancer therapy.[22][23] A Cochrane review found that exercise can also have beneficial effects on health-related quality of life, body image and self-esteem, emotional well-being, sexuality, sleep disturbance, social functioning, anxiety, fatigue, and pain.[24] Positive effects of exercise interventions are more pronounced with moderate- or vigorous-intensity versus mild-intensity exercise programs.[25]
Exercise is safe and feasible in patients undergoing neoadjuvant cancer treatment and surgery.[26] Improved adherence to exercise programs in cancer patients involves setting program goals, prompting practice and self-monitoring, and encouraging participants to generalize behaviors learned in supervised exercise environments to other, non-supervised contexts. Design exercise prescriptions around individual capabilities. Personalize aerobic exercise, frequency, duration, and intensity. Similarly, tailor resistance training, sets, repetitions, and intensity to each individual.[27] For more information refer to the “Prescribing Movement” Whole Health tool.
American Cancer Society Exercise Guidelines
- Achieve and maintain a healthy weight throughout life
- Aim for 150 minutes of moderate-intensity aerobic exercise every week
- Start gradually, if not exercising currently
- Limit time spent sitting
Patients who have never exercised before should start with a 10-minute walk and work their way up. Fatigued patients can split their daily exercise into 10-minute blocks. Use caution in patients with bone metastases and recommend a low-intensity supervised exercise program.
Yoga
Yoga reduces distress, anxiety, and depression in breast cancer patients, and it moderately improves fatigue, quality of life, and emotional and social function, both during and post-treatment.[28] Cancer patients engaging in yoga have consistent improvement in physical symptoms (heart rate, respiratory rate, immunity, fatigue), psychological symptoms (sleep, depression, distress, and anxiety), and quality of life.[29][30][31][32]
Encourage patients to attend yoga classes for patients with cancer or a chronic illness, as the poses and stretches provide appropriate recognition of physical limitations. Yoga allows participants to work at an individual pace and can be modified for unique circumstances, limiting injuries and harm. Participants should be encouraged to listen to their bodies and not attempt poses uncomfortable or strained poses. The amount of yoga practice necessary to yield positive effects has not yet been determined, and benefits vary from engaging in group sessions to following an instructor on a video watched at home.
Currently, there are no evidence-based guidelines specifying contraindications for yoga for patients with cancer. Patients with symptomatic anemia, postural hypotension, and light-headedness should avoid prolonged standing poses and transition slowly between positions. Patients with balance concerns may modify standing poses by using a chair or wall. Patients with fever, systemic infection, or thrombocytopenia should avoid yoga until their condition improves. During neutropenia, patients should avoid group classes. Recommend that patients purchase their own mats to reduce the risk of exposure to infection. Patients should describe physical limitations or concerns to the yoga instructor and inquire about modifications, availability of props, class size and duration, and suitability for their level of skill and physical ability.[33]
Qi Gong
Qi gong is a general term for a large range of Chinese energy exercises and therapies. “Internal” qi gong is self-directed and involves the use of movements, meditation, and control of breathing pattern. The gentle movements and postures of the exercise are designed to achieve a harmonious flow of qi energy in the body to improve physical fitness and overall well-being. “External” qi gong is performed by a trained practitioner using his or her hands to direct emitted qi energy onto the patient’s body for the purpose of diagnosing and treating various diseases. Cancer patients using qi gong exercise in combination with conventional methods have significant reduction in inflammatory markers, 5-year survival rate, fatigue, and cancer-related symptoms. They also have improved blood cell antioxidation capacity.[34] Qi gong positively impacts cancer-related quality of life, survival rate, fatigue, distress, sleep, immune function, and leukopenia.[35][36][37]
Surroundings
Music Therapy
Music therapy is the clinical use of music interventions to address physical, social, emotional, and cognitive needs of a person within a therapeutic relationship facilitated by a credentialed professional. A Cochrane review found that music interventions may benefit anxiety, pain, mood, and quality of life in people with cancer, with further effects on heart rate, respiratory rate, and blood pressure.[38] Within a cancer center, a music therapy program can be used to promote patient self-expression, assist patients with music choices to enhance mood and reduce stress, facilitate group interactions with music serving as the catalyst, and promote physical and emotional well-being. Passive music therapy has been shown to reduce anxiety during radiation, chemotherapy, and post-surgery. It is affordable, easy to implement, non-invasive, and does not have any negative effects.[31]
Environmental Exposures: Carcinogens and Pesticides
Polychlorinated biphenyls. Polychlorinated biphenyls (PCBs) are organochlorides listed as probable human carcinogens by the Environmental Protection Agency. The different forms have various effects, including estrogen modulation, immunotoxicity, and induction of various enzyme systems.[39] Increasing evidence supports a strong causal association between exposure to PCBs and non-Hodgkin’s lymphoma (NHL).[40][41] However, other reviews have concluded that there is no relationship between PCB exposure and various cancers including prostate, testicular, breast, intestinal, and NHL.[42][43] They argue that PCB exposure in rodents leads to a higher burden of the chemical compared to humans due to differences in phase I liver enzyme activation.
Pesticides. Pesticides and herbicides are used on farm crops, lawns, and gardens, and they are also found in household products, including cosmetics. There is a positive relationship between exposure to pesticides and development of some cancers, particularly brain, prostate, kidney, NHL, and leukemia. Studies in pediatric patients indicate an increased risk of cancer associated with prenatal and postnatal exposure, as well as parental exposure at work.[44] A longer period of exposure leads to a higher risk.[45]
Military Exposures
Agent Orange. Agent Orange (AO) is a commercial manufactured herbicide that was sprayed extensively during the Vietnam War. During the manufacturing process, it unintendedly was contaminated with the potent carcinogen TCDD, a dioxin. The Institute of Medicine has concluded that there is sufficient evidence to link AO exposure to soft-tissue sarcoma, NHL, Hodgkin’s lymphoma, and chronic lymphocytic leukemia. There is limited evidence to link AO exposure to respiratory cancers, multiple myeloma, and prostate cancer.[46] A recent study of 2,720 Veterans who underwent biopsy, revealed that AO exposure was associated with a 52% increase in the overall risk of detecting prostate cancer. Additionally, there was a 75% increase in the risk of high-grade prostate cancer, and a two-fold increase in the risk of prostate cancer with a Gleason score ≥ 8 in Veterans exposed to AO.[47] A larger study of 13,144 Veterans reached the same conclusions.[48] The risk increases in people with higher TCDD levels or with increasing time spent in Southeast Asia.[49] In those who have had a radical prostatectomy, AO exposure is associated with a shorter PSA-doubling time after recurrence.[50]
Radiation. Uranium ammunition was used during the Persian Gulf War and in the Balkans; this has caused concern about uranium radiation exposure for Veterans who served there. The exposure risk comes from inhalation, ingestion, and the contamination of wounds with shrapnel or dust containing the material. Being enclosed in a tank carrying uranium ammunition would result in less than 25% of the U.S. occupational ionizing radiation exposure limit and would require 250 hours of direct skin contact to exceed the limits for skin. Several reviews have concluded that, although there is a potential for uranium exposure to cause lung cancer, the risk of harm following depleted uranium exposure is low.[51][52][53][54] The highest mean lifetime risk for lung cancer for a scenario with the longest exposure time of 2 hours was only 0.42%, compared with the background risk of 7.35%.[55]
Personal Development
Positive Thinking
Positive thinking involves the use of mental techniques and strategies to overcome unpleasant, unwanted, and destructive attitudes and states of mind. Cancer patients can use positive mental imagery to influence the body. However, the societal tendency to downplay the negative and emphasize the positive trivializes the legitimacy of a person’s circumstances and the stress they cause. False optimism prevents people from expressing negative feelings, since it results in others being unwilling or unable to hear about them.[56] Attempted suppression of negative thoughts and emotions can harm one’s physical health and may decrease immune functioning. Many cancer survivors attribute non-reoccurrence to having a positive attitude, and those who do experience reoccurrence may feel they were not positive enough. It is critical to explain to patients that coping strategies such as positive thinking, having hope, or trying to maintain normalcy may not directly influence their actual control over or responsibility for their cancer outcomes.[57]
Food and Drink
The Standard American Diet (SAD) is low in nutrients and high in inflammatory components including animal fat and chemicals used for processing. In this section, we will discuss the impact of weight, macronutrients (fiber, fats, carbohydrates), and specific foods on cancer.
Weight
Obesity is defined as a body mass index (BMI) greater than 26. The prevalence of obesity is rising, as demonstrated by the Centers for Disease Control and Prevention’s (CDC) Behavioral Risk Factor Surveillance System, and it is contributing to the increased incidence of metabolic syndrome and chronic diseases that accompany it.
The largest prospective analysis on the weight and cancer relationship conducted by the American Cancer Society found that adulthood obesity was associated with increased mortality from colon, breast, endometrial, kidney, esophageal, gastric, pancreatic[58], prostate, gallbladder, and liver cancer. This study estimates that 14% of all cancer deaths in men and 20% in women are attributed to being overweight or obese. Those who were severely obese with a BMI greater than 40 had a 52% and 88% risk of all cancer death for men and women, respectively, compared to people of normal body weight.[59]
Analysis of the Nurses’ Health Study showed that a 5%-10% weight gain within 5 years of a breast cancer diagnosis related to 50% higher rates of breast cancer recurrence and death.[60] Women with invasive breast cancer who have a BMI greater than 25 are 2.5 times as likely to die of their disease within 5 years of diagnosis compared with those who have a BMI less than 21.[61] For patients with colon cancer, a BMI greater than 35 at diagnosis is associated with a 38% and 49% increased risk of recurrence or death, respectively, compared with a BMI less than 25.[62] A BMI greater than 35 is associated with a twofold increase in prostate cancer mortality compared with BMI less than 25.[63] Adherence to BMI recommendations can vary from 34%-77% after completion of cancer treatment, so this should be given particular emphasis.[64]
The mechanisms contributing to tumor promotion include increases in levels of insulin-like growth factor, insulin, leptin, and pro-inflammatory cytokines. The cytokines promote cellular proliferation, inhibit apoptosis, and stimulate growth, migration, and invasion of cancer cells.[65][66]
Patients should maintain a BMI of 21-26 to reduce the risk of getting cancer or achieve that goal once diagnosed to reduce cancer morbidity and recurrence.
Fiber
The SAD tends to be low in fiber. In general, soluble fiber (e.g., oat bran, flax, psyllium) slows blood glucose absorption and insoluble fiber (e.g., nuts, vegetables, whole wheat) makes bowel movements more regular.
There is a 7% reduction in breast cancer risk for every 10 gm per day increment in dietary fiber intake.[67][68] A 2013 prospective study of 11,576 of invasive breast cancer patients confirmed that a diet rich in dietary fiber from vegetables may be associated with a small reduction in risk of breast cancer.[69]
A high intake of dietary fiber from fruits and vegetables is associated with a reduced risk of colorectal cancer.[70] Fiber intake is also associated with a reduced risk of developing pancreatic cancer,[71] esophageal cancer,[72] head and neck cancer,[73] and renal cell cancer.[74]
Patients should eat 25 gm or more of dietary fiber per day, with fruits and vegetables as the major source.
Fats
There is a weak relationship between total fat intake and prostate and ovarian cancer incidence.[75][76] There is no correlation between dietary fat intake and the incidence of breast,[77] esophageal, and gastric cancers.[78] The Women’s Intervention Nutrition Study found that breast cancer patients who initiated a low-fat diet of less than 31 gm of fat per day had a reduced risk of relapse.[79]
High consumption of foods containing omega-3 fats such as olive oil, walnuts, avocados, and cold-water fish and low consumption of omega-6 fats reduces the overall incidence of cancer,[80] in particular colon, prostate, and breast cancer.[81] A recent, oft-criticized study suggests that saturated fat, alpha-linolenic acid (ALA), and eicosapentanoic acid (EPA) intake is related to increased risk of advanced or fatal prostate cancer;[82] however, the overall health benefits of omega-3 fats outweigh recommending against their intake. Omega-3 fats may exert their anticancer actions by influencing cell proliferation, cell survival, angiogenesis, inflammation, metastasis, and epigenetic abnormalities.[83]
Patients should increase intake of omega-3 fats and reduce the intake of saturated and trans fats to reduce the risk of developing cancer.
Red Meat
Red and processed meat (deli meat, hot dogs, sausage) consumption correlates with an increased risk of gastric,[84] colon,[85][86][87][88] esophageal,[89] pancreatic,[90] breast[91], and lung cancer.[92] There is no known correlation between red meat consumption and kidney,[93] ovarian,[94] breast, or prostate cancer.[95][96]
Patients should limit intake of red meat, particularly processed meat, to no more than 1-2 servings per week.
Soy
Soy foods (miso, tempeh, soybeans, tofu, soy milk) have received attention regarding their influence on cancer, particularly breast cancer. They contain isoflavones, which consist of phytoestrogens and selective estrogen receptor modulators, namely genistein and daidzein. On average, a typical serving of soy (250 gm of soymilk, 100 gm of tofu) contains 20-30 mg of soy isoflavones per serving.
In a 2013 meta-analysis, post-diagnosis soy food consumption was associated with reduced mortality and recurrence in breast cancer patients,[97] and when comparing the highest isoflavone intake to the lowest, mortality was reduced 17% and recurrence was reduced 25%.[98] Soy consumption reduced recurrence to a greater extent in tamoxifen users, estrogen-receptor negative patients, and postmenopausal women.[98] However, prediagnostic soy consumption is not related to decreased mortality from breast cancer in postmenopausal women.[99][100]
Soy consumption is associated with a reduced risk of gastric and colorectal cancer in women.[101][102] Isoflavone-containing foods are associated with a reduced risk of endometrial cancer in postmenopausal women.[103][104] Soy food intake may reduce prostate cancer risk.[105][106] Prediagnosis soy consumption is associated with better survival in women with lung cancer.[107]
Patients should eat 1-2 servings of soy foods (preferred over dietary supplements) daily, especially women.
Cruciferous Vegetables
Cruciferous vegetables are in the Brassica family, which includes broccoli, cauliflower, cabbage, bok choy, kohlrabi, kale, arugula, horseradish, mustard greens, turnip greens, collard greens, and watercress, among several others. Their consumption is associated with a reduced incidence of prostate, breast, colon, and bladder cancer.[108][109][110]
This protective effect is attributed to isothiocyanates and indoles, namely sulforaphane and indole-3-carbinol, present in these vegetables, which act on the molecular level to decrease oxidative stress, induce apoptosis, suppress cell cycle progression, and inhibit angiogenesis.[109][111] These phytochemicals become more bioavailable when cruciferous vegetables are cut, cooked, frozen, pressurized, or thoroughly chewed.
Patients should eat at least 3-4 servings of fruit and 5-6 servings of vegetables daily, 1-2 of which are from cruciferous vegetables.
Mediterranean Diet
A Mediterranean diet (MD) is low in saturated and omega-6 fats (meat, poultry, and dairy), and high in plant sources of monounsaturated fats, omega-3 fats, fiber, antioxidants, and polyphenols (fruit, vegetables, olive oil, and wine). It is associated with a reduction in cancer mortality.[112] Adherence to an MD excluding alcohol is related to a modest reduced risk of breast cancer in postmenopausal women,[113] but another study done the same year found no effect.[114] Eating the MD also correlates with a reduction of colorectal[115] and pancreatic cancer risk.[116] The MD is not associated with the risk of advanced prostate cancer or disease progression. However, greater adherence to the MD after diagnosis of nonmetastatic prostate cancer was associated with a 220% lower risk of overall mortality.[117]
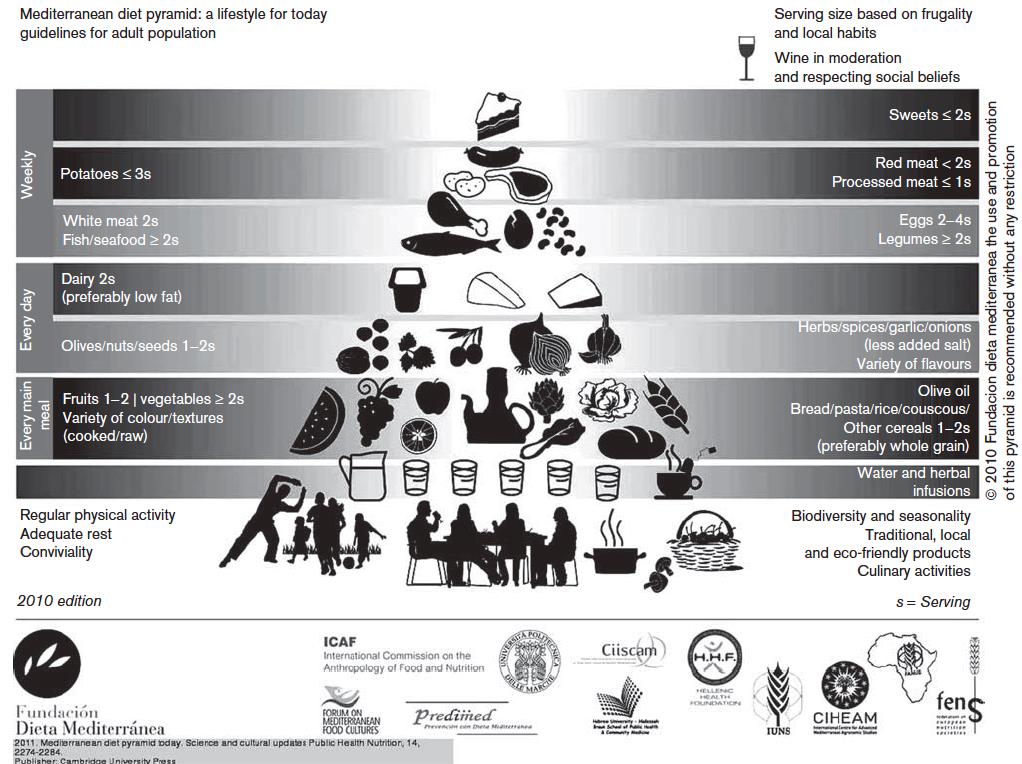
American Cancer Society Nutrition Guidelines
- Eat a healthy diet with an emphasis on plant foods
- Limit intake of processed and red meat
- Eat at least 2 1/2 cups of vegetables and fruit daily
- Choose whole grains instead of refined grains
Recharge
Sleep
Sleep disruption leads to weight gain, metabolic syndrome, and cardiovascular disease. Sleep problems are prevalent in night workers and shift workers. Sleep deprivation impacts heavily upon the entire neuroendocrine-immune system complex that regulates cell proliferation, immune defense (including cytokine production and associated proinflammatory pathways), energy metabolism, and the biological response and adaptation to everyday stresses.[119] There is an increased risk of breast cancer in women who work a night shift or rotating shift for at least half a year within 5 or more years preceding their diagnosis.[120] A meta-analysis of prospective cohort studies found a 38%-72% decrease in breast cancer for longer sleepers. The same study found a 34% decrease in breast cancer risk in people with the highest urinary melatonin metabolite concentration; people who make more melatonin seem to have lower breast cancer risk.[121] However, a more recent meta-analysis concluded that sleep duration has no effect on breast cancer risk.[122]
Encourage patients to aim for 7-8 hours of sleep daily.
Family, Friends, and Co-Workers
Support Groups
Support groups can be very helpful for numerous conditions and are commonly available for cancer survivors. Support groups have a leader who facilitates the group and encourages the sharing of experiences among participants during planned activities. Support groups offer practical help, emotional support, and provide positive feedback. Cancer-related support groups provide a high level of satisfaction. They also improve pain, morale, and quality of life.[123][124] One review found that one-on-one, face to-face, and group Internet programs should be given priority as ways to offer peer support.[125] The most effective cancer-related support groups provide people with the tools needed to manage the cancer diagnosis, in addition to an emotional outlet. These tools can range from cooking classes to mind-body techniques such as meditation.
Spirit and Soul
Spirituality and prayer are primary strategies for coping with the stress of cancer survival. It is essential for clinicians to screen for spiritual distress, identify spiritual needs, and facilitate appropriate spiritual care at several time points in patients with advanced cancer.[126] Religious and spiritual beliefs aid people in accepting their illness and cope with it in a positive and purposeful way. They also influence patients’ decision-making regarding integrative therapies and end-of-life care. Religious beliefs remind people of their identity, give them hope, meaning, and purpose, and allow for connectedness. Unfortunately, the majority of people feel that their spiritual needs are unaddressed during their treatment. The benefits of providing spiritual support include reducing stress and anxiety, improving one’s sense of belonging, strengthening the will to live, and promoting inner peace.
Power of the Mind
For many people, the diagnosis of cancer is a deeply stressful and emotional experience, which often causes anxiety, depression, and anger. People often fear the diagnosis because of its associated mortality risks, as well as the side effects of treatments. Depending on where a given person with cancer is, in the process of diagnosis and treatment, different concerns will come to the forefront. These concerns can interfere with the patient’s overall well-being and effective participation their own healing process. Mind-body interventions are important for helping patients optimize their course to recovery, in part because they enhance the mind’s capacity to affect bodily functions.
Meditation
Meditation is a mind-body technique that trains the mind in awareness and attention through mental focus on an object, sound, word, phrase, or the breath. Mindfulness meditation involves attending to the whole of an experience nonjudgmentally, without a sense of subject and object. Its primary benefits include relaxation, psychological insight, and decreased stress and pain. Mindfulness-Based Stress Reduction (MBSR) is the most studied form of meditation for cancer patients, and it is a structured 8-week group program of weekly 2.5-hour sessions and one all-day silent retreat. Cancer patients who train in MBSR experience less tension, anger, depression, anxiety, fear, emotional instability, habitual stress behavioral patterns, and fewer concentration problems, and improved immune function.[127][128] MBSR significantly reduces anxiety and depression, and promoted sleep quality in patients with cancer, with effects maintained up to 6-12 months after the course.[31][129][130][131] For more information, refer to the “Mindful Awareness” Whole Health overview.
Clinical Hypnosis
Hypnosis is a natural state of aroused, attentive focal concentration coupled with relative suspension of peripheral awareness and aims to achieve symptom relief. Relaxation can pair with imagery to reduce the distressing symptom. In cancer patients, clinical hypnosis is effective for reducing pain, anxiety, depression, anticipatory or chemotherapy-related nausea and vomiting, procedural-related pain and distress, and hot flashes.[127] Cancer care health care providers should pay attention to what their words may be suggesting to patients, whether or not a formal hypnotic induction is used. Negative suggestions include ‘‘little sting here’’ or ‘‘sharp scratch there’’ while more neutral descriptors are ‘‘some feeling of warmth, coolness, or tingling.’’
Guided Imagery
Guided Imagery involves engaging the imagination to create a sensory experience to achieve a clinical goal. The goal can be specific, such as reducing pain, or general, such as achieving emotional well-being. Often, if coupled with progressive muscle relaxation, it induces the relaxation response. Guided Imagery has been found helpful for anticipatory and chemotherapy-related nausea and vomiting; reducing anxiety, depression, and discomfort; and improving quality of life.[128][132] Guided Imagery CDs specific to the various phases of cancer care, including radiation and chemotherapy, have been created by Belleruth Naparstek and can be found at the Health Journeys website, which offers discounts to Veterans.
For further information, refer to “Colorectal Cancer Care and Prevention” and “Lung Cancer Care and Prevention.”
Conventional Approaches
The following is a brief overview of the conventional therapies used in cancer care.[133] A full evidence-based review is beyond the scope of this overview.
Surgery
Solid tumors that have not metastasized are removed surgically with clear margins to prevent their spread. Sometimes, surgery can be all that is required. In other cases, surgery is the first part of an overall plan that may include chemotherapy, radiation, or other treatments. Depending on the extent of surgery, the recovery period can range from a couple days to weeks requiring rehabilitation or subsequent surgeries. To ease fears of patients undergoing surgery, help them become comfortable about the procedure, with the surgeon, and where it will take place.
Radiation
Radiation can be used to shrink tumors prior to surgery, eliminate a tumor, help prevent a recurrence, or in palliation to relieve symptoms caused by cancer.[134] Often it is recommended with surgery for localized cancers or those in distinct locations in the body. Radiation is a form of ionizing energy delivered through a beam targeted to a tumor or group of cells; the ions trigger cellular apoptosis. Brachytherapy delivers radiation in or near the tumor through pellets placed into the tissue. Side effects of radiation may include anemia, fatigue, gastrointestinal distress, hair loss, skin irritation, and burns. More-serious side effects include fibrosis, secondary cancers, and bone marrow damage.
Chemotherapy
Chemotherapy can be used to help cure cancer, control cancer, and ease symptoms of the disease.[135] It is used for cancers not sensitive to radiation or too widely spread. It is the primary treatment for lymphoma, leukemia, and metastasized solid tumors. It is also used to prevent recurrence in early stages of the disease for some types of cancers (e.g., breast) and can be used to shrink tumors prior to surgery. Chemotherapy involves injecting or orally taking chemical agents that are toxic to cells with the goal of halting reproduction and growth. Side effects vary based on the drug and the patient’s sensitivity to it, and may include gastrointestinal distress, hair loss, mouth sores, fatigue, insomnia, anemia, infection, neuropathy, and cardiovascular damage.
Targeted Therapies
These include tyrosine-kinase inhibitors, monoclonal antibodies, and hormonal therapies. Targeted treatment strategies interact with their specific targets to prevent cell division or trigger apoptosis. They are generally better tolerated than chemotherapy, but side effects include rash, gastrointestinal distress, myalgias, and elevated blood pressure.
Complementary Approaches
Massage
Massage Therapy induces relaxation from stress, and the National Comprehensive Cancer Network (NCCN) now recommends massage in its ‘‘Guidelines for Supportive Care,’’ based on the growing body of evidence of massage safety and benefits for quality of life. Swedish massage for cancer patients can alleviate a wide range of symptoms including pain, nausea, anxiety, depression, anger, stress, and fatigue.[136] A Cochrane review concluded that massage improves psychological well-being by reducing pain and relieving anxiety.[137] The most commonly used protocol of classic massage is twice weekly 30-minute sessions for at least 5 weeks immediately following cancer treatment.[31]
Massage therapists should have additional knowledge, skill, and experience in safely practicing with cancer patients, which requires specialized training in oncology massage. Typically, pressure should be lighter over bone metastases, areas with peripheral neuropathy, surgical scars, sites of deep vein thrombosis risk, and areas of cancer pain and discomfort. Overall pressure is modified for patients with thrombocytopenia, leukemia, or whenever easy bruising and bleeding are likely. To avoid precipitating a lymphedema episode or triggering chronic lymphedema, oncology massage therapists are careful to avoid heat, pressure, and excessive joint movement in the body region served by the missing or compromised lymph nodes.
Chinese Medicine
Chinese medicine (CM) encompasses the use of herbs, moxibustion, and acupuncture, among many other techniques, to individualize therapy for each person. CM can be very effective at reducing the side effects of cancer treatment including pain, nausea, anxiety/depression, and neuropathy.
For further information on incorporating CM into cancer care, refer to the “Managing Side Effects of Chemotherapy and Radiation” Whole Health tool.
Acupuncture
Acupuncture is an integral part of Chinese medicine and can reduce common side effects experienced by cancer patients undergoing treatment leading to an improved quality of life. Acupuncture can significantly improve pain, chemotherapy-induced nausea and vomiting, cancer-related fatigue, xerostomia (dry mouth), anxiety, depression, and sleep. Nonmedical doctors and physicians can provide acupuncture, but they should have specific training in oncology. Precautions should be taken to prevent bacterial contamination, avoid limbs with lymphedema, and apply needles to easily compressible sites in patients with a bleeding tendency. Six to eight sessions are needed to determine whether acupuncture is effective for the patient.[138][139]
For further discussion on acupuncture’s role in the management of cancer treatment side effects, refer to the “Managing Chemotherapy-Induced Nausea and Vomiting,” and “Managing Side Effects of Chemotherapy and Radiation” Whole Health tools.
Dietary Supplements
Note: Please refer to the Passport to Whole Health, Chapter 15 on Dietary Supplements for more information about how to determine whether or not a specific supplement is appropriate for a given individual. Supplements are not regulated with the same degree of oversight as medications, and it is important that clinicians keep this in mind. Products vary greatly in terms of accuracy of labeling, presence of adulterants, and the legitimacy of claims made by the manufacturer.
Vitamin D
High vitamin D levels are associated with a lower risk of breast,[140][141][142][143], colorectal,[144][145][146] thyroid,[147][148] bladder,[149][150][151] and lung[152][153] cancers. Higher levels of circulating vitamin D reduce breast cancer mortality in a dose-dependent manner.[147] Several studies indicate that vitamin D levels do not correlate with prostate,[154][155][156][157] esophageal,[158] or gastric cancers risk.[159] A study of Veterans with bladder cancer found that a higher vitamin D level was linked to longer survival.[160] A large systematic review and meta-analysis found that vitamin D supplementation does not reduce the incidence of cancer or cancer mortality.[161] Some foods (eggs, fortified dairy, mushrooms, and fish) provide small amounts of vitamin D2 (ergocalciferol), but ultraviolet light from the sun is the best source of vitamin D3 (cholecalciferol). Vitamin D production is impaired with age, obesity, and pigmentation, so oral supplementation is advised.
Dose: Each 1000 IU of vitamin D3 should increase 25-hydroxyvitamin D levels by 10 ng/ml. A safe recommendation is to achieve a 25-hydroxyvitamin D level in the 50-80 ng/ml range.
Antioxidants
The use of antioxidants (vitamins A, C, E, selenium, and carotenoids) during cancer treatment is controversial due to the theoretical risk of reducing the number of free radicals created by chemoradiotherapy. Since free radicals target and kill cancer cells, suppressing production can reduce the effectiveness of cancer treatment. Antioxidants have not been found to prevent gastric,[162] skin,[163] prostate,[164] or colon cancers.[165] Other meta-analyses found that antioxidants do not prevent any cancer.[166][167][168] Beta-carotene increases lung cancer risk in people who smoke and have asbestosis.[169] Preliminary evidence suggests that high-dose intravenous antioxidants during chemotherapy can reduce side effects and potentiate treatment effects, but that is beyond the scope of this discussion as specific therapeutic recommendations cannot yet been made.
Do not recommend that your patients start on antioxidants to prevent cancer, but rather encourage a high intake of fruits (especially berries and dark grapes) and brightly colored vegetables to obtain them naturally.
For more information, refer to the “Supplement/Botanical Interactions with Chemotherapy and Radiation” Whole Health tool.
Curcumin
Curcumin is the major component of the Indian spice turmeric (Curcuma longa) and has anti-inflammatory and chemopreventative properties. Curcumin inhibits COX-2 activity and TNF-alpha signaling pathways and regulates the expression of p53 tumor suppressing gene.[170] Curcumin has reported activity against leukemia and lymphoma, gastrointestinal cancers, pancreatic cancer, genitourinary cancers, breast cancer, ovarian cancer, head and neck squamous cell carcinoma, lung cancer, melanoma, neurological cancers, and sarcoma.[171] It has also been shown to protect normal organs such as liver, kidney, oral mucosa, and heart from chemoradiotherapy-induced toxicity.[172]
Dose: 500-3,000 mg daily combined with piperine (black pepper) to increase bioavailability and taken during meals containing healthy fats to enhance absorption.
Green Tea
Green tea consists of unfermented Camellia sinensis tea leaves with a high polyphenol content, 40% of which is epigallocatechin gallate (EGCG). Green tea consumption is associated with a reduced incidence and recurrence of breast cancer.[173][174][175][176] Green tea may have protective effects against developing primary prostate cancer[177][178][179][180] and non-Hodgkin’s lymphoma,[181] with mixed evidence for gynecologic,[182][183][184][185][186][187] esophageal,[188][189] gastric,[190][191] and liver cancers,[192][193][194][195] and no evidence for colorectal cancer.[196][197]
Dose: Recommended green tea intake is 3 to 5 cups per day (up to 1,200 ml daily), providing a minimum of 250 mg daily catechins. Liver enzymes should be monitored in patients taking high-dose green tea supplements.
Interactions: May inhibit the enzyme CYP450 3A4 (needed to help remove foreign substances from the body) if using more than 800 mg daily, and may increase tamoxifen bioavailability.[171]
Melatonin
Melatonin is a hormone secreted by the pineal gland that regulates sleep patterns. Its actions include stimulating growth hormone production, triggering apoptosis, up-regulation of antioxidant enzymes, suppression of tumor and endothelial growth factors, and down-regulation of pro-oxidative enzymes.[171] There is an improved effect of chemoradiotherapy and 1-year survival rates among cancer patients with complete response, partial response, and stable disease using melatonin as adjuvant treatment. Melatonin significantly reduce fatigue, neurotoxicity, asthenia, leukopenia, nausea and vomiting, hypotension, and thrombocytopenia from chemotherapy.[198][199]
Dose: The adjuvant dose is 20 mg at night, and it can cause sedation, vivid dreams, and headaches. For insomnia, the dose is 1-10 mg at night. The effective dose varies greatly from one person to another, in terms of sleep effects.
For more information, refer to the “Mycomedicinals (Mushrooms) for Cancer” and “Supplement/Botanical Interactions with Chemotherapy and Radiation” Whole Health tools.
Personal Health Plan
Name: Pam
Date: xx/xx/xxxx
Mission, Aspiration, Purpose (MAP):
My mission is to get through breast cancer treatment with minimal side effects while improving my health, and to become more in touch with my spirituality and find inner peace.
My Goals:
- Start running 3 times a week.
- Learn how to cook meals at home to improve nutrition and lose 30 lbs.
- Clear the clutter by having a more-structured daily schedule, allowing enough time for sleep, family, and myself.
Strengths (what’s going right already)/Challenges:
My Plan for Skill Building and Support
Mindful Awareness:
- Pay more attention to when I am pushing my limits too much, whether it is at work or at home. Engage my sons in helping take some of the load off my responsibilities at home.
- Run, to clear the clutter in my head and move from stressful thought patterns to healthier, more emotionally sound ones.
- Keep a gratitude journal every night and write one thing in it that I am grateful for.
Areas of Self-Care:
- Moving the Body
- Start running regularly and aim for 3 hours per week. Try getting my sons into it as well, to train for a half marathon together in the upcoming months. To work on the mind-body aspect of exercise, try a free tai chi class at the cancer resource center.
- Surroundings
- Work with my sons to keep the house tidy and free of clutter. Speak with my Primary Care Physician about ways to quit smoking when I am ready.
- Personal Development
- Join a knitting group to socialize with new people. To achieve work-life balance, start thinking about giving up one of my board positions at the hospital.
- Food and Drink
- Follow an anti-inflammatory diet by minimizing refined sugars and processed foods in my diet. Take a healthy cooking class with my sons so that we can cook together.
- Recharge
- Aim for 8 hours of sleep a night. Try to minimize night shifts at work. Stop drinking caffeine after 12 pm.
- Family, Friends, and Co-Workers
- Do more activities with my sons. In addition, use this time to get closer with my mother.
- Spirit and Soul
- Explore finding a spiritual anchor through mindfulness meditation. Join an 8-week Mindfulness-Based Stress Reduction course to help through this journey. Alternatively, I can start by listening to the audiobook “Mindfulness for Beginners” by Jon Kabat-Zinn and reading his book “Full Catastrophe Living.”
- Power of the Mind
- Start with deep breathing exercises at night to help relax before going to bed. Maybe seek the help of a therapist to address anxiety and worries.
Professional Care: Conventional and Complementary
- Prevention/Screening
- Up-to-date on PAPs, screening labs, and immunization
- Treatment (e.g., conventional and complementary approaches, medications, and supplements)
- Start treatment plan per the oncologist.
- Consider massage therapy to help with anxiety.
- Continue current medications.
- Take melatonin 5-20 mg at night to help sleep as this also has anti-cancer properties
- Skill building and education
- Mindfulness-based stress reduction, breathwork, and Guided Imagery resources
- Anti-inflammatory diet
Referrals/Consults
- Oncology rehab therapy to prevent lymphedema and cording postoperatively, or to treat neuropathy from chemotherapy
- Psychologist for counseling
- Massage therapist
Community
- Support groups through the VA health system or American Cancer Society
Resources
- Meditation phone apps: Calm, Headspace
- Books: Mindfulness for Beginners, by Jon Kabat-Zinn
My Support Team
- Principal Professions
- Primary Care Clinician
- Oncologist
- Psychotherapist
- Personal
- Best friend, Anne
- Sons, Mike and Nick
- Co-worker, Linda
Next Steps
- Sign up for cooking class.
- Keep a food and exercise log to meet my
- Follow up with an oncologist as scheduled.
- Follow up with the team in 1-2 months.
Please Note: This plan is for my personal use and does not comprise my complete medical or pharmacological data, nor does it replace my medical record.
Follow-Up With Pam
In the past two months, Pam has started running 5 times a week in the morning before she goes to work. After much deliberation, she decided to give up one of her board positions at the VA so she could have more time for herself and her family. She and her sons love spending time cooking dinner together. She has made some new friends at her weekly knitting sessions and uses it as therapy to help reduce her anxiety. The melatonin has helped her sleep better, and she has stopped smoking with the use of smoking cessation medication.
She is receiving chemotherapy treatments currently and experiencing a significant amount of nausea. But she uses ginger and peppermint tea to help control her symptoms, along with the prescribed anti-nausea medications. She is also taking supplements to enhance the effectiveness of the chemotherapy and reduce side effects like neuropathy and fatigue. Overall, she has a much more positive attitude, and the breast cancer diagnosis has given her a new outlook on life.
Next month, she hopes to join a local MBSR course to learn mindfulness meditation. Now that she has more time in her life, she also will be reconnecting more with her mother. She is thankful that she has been able to work with you to reach her health goals during a very stressful time in her life.
Whole Health Tools
Resources
Organizations
- National Center for Complementary and Alternative Medicine (NCCAM)
- For information on complementary approaches
- National Cancer Institute
- Refer to the section on Complementary and Alternative Medicine
- Office of Cancer Complementary and Alternative Medicine (OCCAM)
- American Cancer Society
- National Comprehensive Care Network (NCCN)
- Society for Integrative Oncology
- Memorial Sloan Kettering Cancer Center
- Evidence-based information about herbs, botanicals, supplements, and other products
- MD Anderson Cancer Center
- Integrative Medicine Program
Exercise Tools
- Moving for Life: a dance exercise program for cancer recovery available for purchase
- Qigong by Ken Cohen (DVD)
- The American College of Sports Medicine (ACSM) in collaboration with the American Cancer Society (ACS) certifies Cancer Exercise Trainers. To locate a Certified Cancer Exercise Trainer in your area, go to the ACSM ProFinder website. Click on ACSM/ACS Certified Cancer Exercise Trainer in the “Certification/Registry Level” box.
- Yoga for Cancer by Tari Prinster
Author(s)
“Cancer Care” was written and updated by Srivani Sridhar, MD (2014, updated 2020).



















