Whole Health emphasizes mindful awareness and Veteran self-care, in addition to excellent professional care. The Circle of Health highlights eight areas of self-care: Surroundings; Personal Development; Food & Drink; Recharge; Family Friends, & Co-Workers; Spirit & Soul; Power of the Mind; and Moving the Body. The narrative below shows what a Whole Health clinical visit could look like and how to apply the latest research on complementary and integrative health (CIH) to digestive health.
A Whole Health approach to digestive health also incorporates complementary and integrative practices to prevent or improve acute and chronic symptoms related to Celiac disease, GERD, irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and other gastrointestinal issues.
Meet the Veteran

Jake is a 36-year-old sergeant in the Army who has always had a “sensitive gut.” His symptoms “really got bad” after he required a broad-spectrum antibiotic for a pneumonia he was treated for in Afghanistan. He also has a history of asthma, and the combination of poor sleep and a stressful mission took its toll. His asthma worsened, and he developed a secondary lobar pneumonia. He started on a 5-day burst of prednisone and 5 days of azithromycin. After his productive cough continued, he then was then given 14 days of Augmentin. He reports that his bowels have never been the same since.
Jake has what many would label diarrhea-predominant IBS, with 5-6 watery stools a day, bloating, and diffuse gas pains. Any food seems to make his symptoms worse, particularly beer, which he enjoys drinking with his friends. His diet is rather poor. He eats processed, “white” foods with few vegetables and fruits. He has been using over-the-counter omeprazole with little change in his symptoms. The frequency of diarrhea seems to get worse as he starts to look for a job. He is worried about supporting his wife and young daughter.
Personal Health Inventory
On his Personal Health Inventory (PHI), Jake rates himself 4 out of 5 for his overall physical well-being and a 2 for overall mental and emotional well-being. When asked what matters most to him and why he wants to be healthy, Jake responds:
“I want to be able to provide for and support my wife and daughter. I love playing with my daughter. I enjoy deer hunting or sometimes just sitting in my tree stand; connecting to nature is more important than filling my tag.”
For the eight areas of self-care, Jake rates himself on where he is and where he would like to be. He decides to first focus on the areas of Food and Drink and Power of the Mind to better control his “broken bowels” and work on his stress levels.
For more information, refer to Jake’s PHI.
Introduction
General Overview: Key Elements of Healthy Digestoin
- Gastrointestinal Ecology
- The Gastrointestinal (GI) environment is in a dynamic balance influenced by exposure to bacteria, nutrition, emotions, and medications.
- Intestinal Permeability
- The integrity of the barrier between the gut and immune system can influence systemic illness and symptoms.
- Key nutrients, positive emotions, and healthy bacteria can improve the integrity of this vital barrier.
- Adequate Acidity
- Prolonged acid suppression can result in a decrease in protease activation that may reduce the digestibility of food proteins.
- Acid is necessary to absorb key nutrients required for a healthy GI ecosystem.
- Establishing the Microbiome
- Bacteria that populate the GI ecosystem are necessary for optimal GI and immune function.
- Facilitating an optimal GI ecosystem can result in healthy bacterial growth.
- Fiber promotes mucus and nutrients that the bacteria need to grow, allowing them to metabolize shortchain fatty acids that help maintain the health of the GI tract.
Gastrointestinal Ecology
Understanding how to facilitate health within the gastrointestinal (GI) system not only requires knowledge of medicine but also an understanding of ecology. The GI system is a dynamic living environment that relies on many interconnected influences to optimize function. In the field of afresh
the term Intermediate Disturbance Hypothesis suggests that a small amount of stress to an ecosystem is healthy. It encourages nature to adapt and strengthen its defenses and promotes resiliency. However, too much stress can overpower these defense mechanisms and send the ecosystem into an imbalance of disrepair.
Jake’s circumstances are an example of how the stressors he encountered overcame his GI ecosystem’s ability to maintain this balance. Our goal is to help bring this dynamic ecosystem back to health.
To understand where to start, we need to review the communication mechanisms that create a healthy barrier between the lumen of the GI tract and the enteric immune system that allows nutrients to enter the circulatory system. The integrity of this system is vital. The enterocytes are covered by mucus and bacteria that are in constant communication with the gut associated lymphoid tissue (GALT) that not only allows nutrients to pass into the blood stream, but can also influence systemic inflammation and disease.
Intestinal Permeability
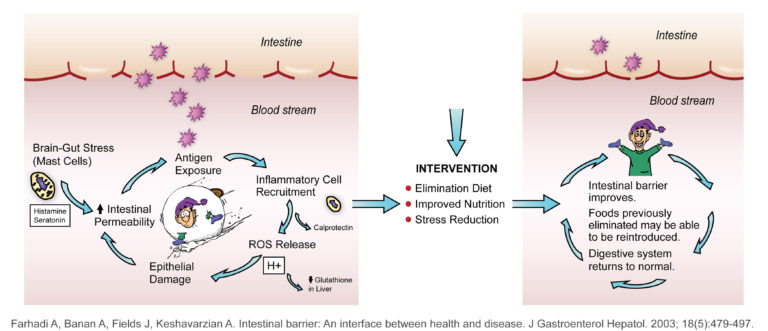
Figure from University of Wisconsin Integrative Medicine depicting information from Farhadi.[ref id=1]
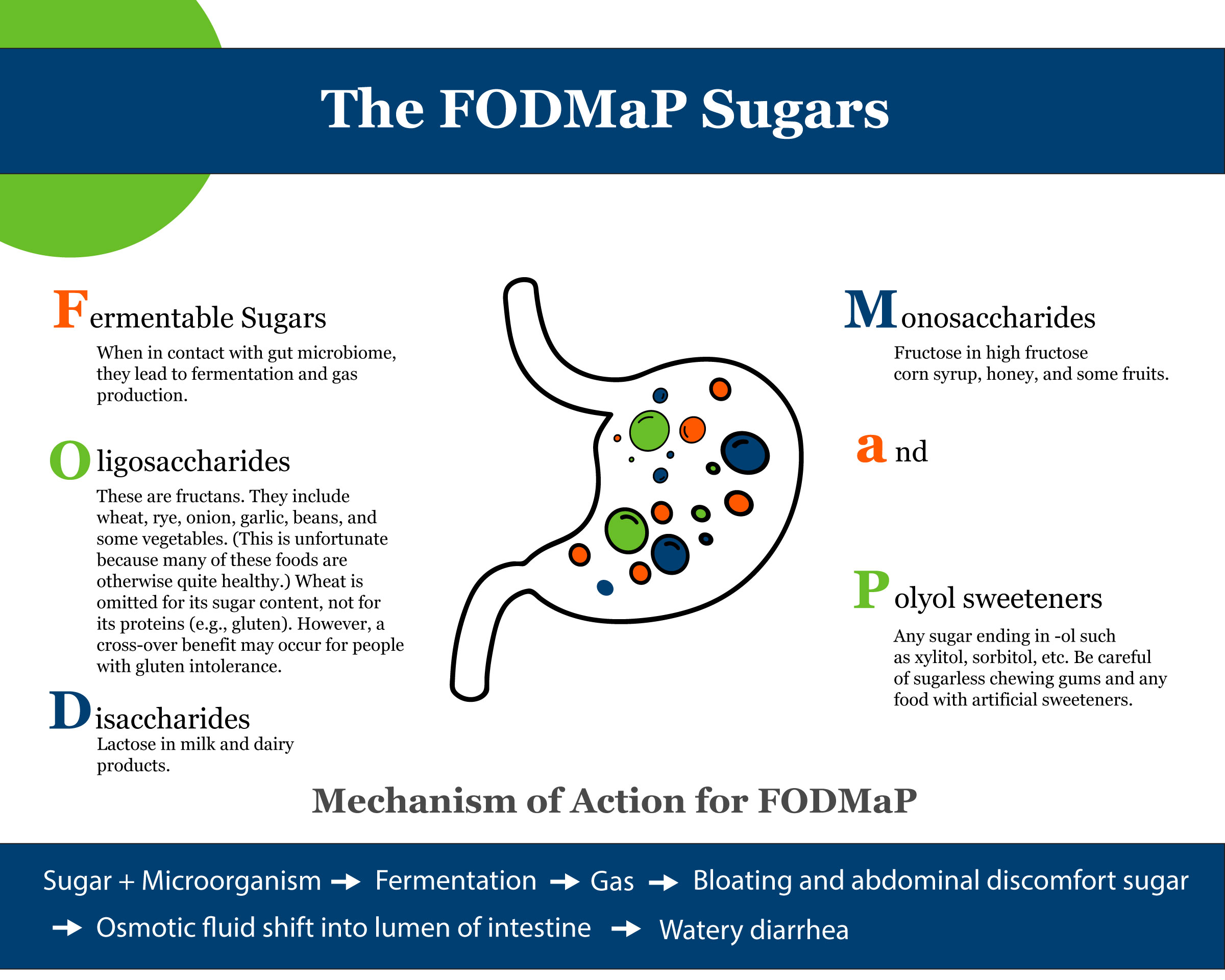
Figure 1 shows how a disruption of this barrier can lead to an imbalance of the GI ecosystem. If the integrity of the GI lining is compromised, food proteins can cross and be recognized by the GALT as foreign, resulting in an inflammatory reaction that can lead to GI symptoms, worsening systemic disease, and production of pro-oxidants. This results in a snowball effect that promotes continued disruption of the ecosystem. This is called, “increased intestinal permeability” in the medical literature and “leaky gut” in the lay press.[1] An example of this process can be seen in Celiac Disease and Non-Celiac Gluten Sensitivity. To differentiate between the two, refer to the section below.
Both nutritional and emotional factors can influence whether one develops a “leaky gut.” With the perception of stress, mast cells release histamine and serotonin that can encourage breakdown of the intestinal barrier. This is why it is so important to address both physical and emotional aspects of this ecosystem. Thus, in order to enhance the health of this barrier and reduce intestinal permeability, a Whole Health approach is key.
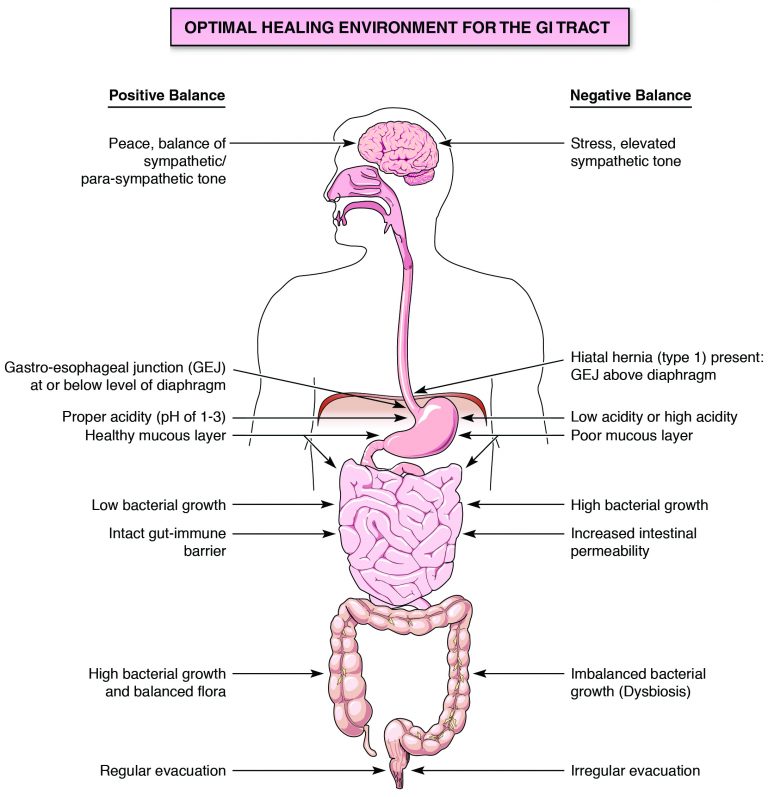
Figure 2 compares the elements of an optimal GI environment and one that is unhealthy. It explores factors that positively and negatively affect GI health.
Adequate Acidity
One of the key ingredients for an optimal GI ecosystem is an adequate gastric pH, which should fall in the 1-3 range. Many individuals may have chronic reflux with esophageal changes, placing them at risk of adenocarcinoma and making long-term acid suppression a requirement for them. Many others may inappropriately utilize these medicines to chronically suppress symptoms, which is not consistent with the Whole Health approach. There are many potential risks to long-term acid suppression. To learn more, read to the “Coming of a Proton Pump Inhibitor” Whole Health tool.
Figure 3 shows how an acidic environment activates pepsinogen to create protease that helps break down proteins. If the pH is too high (greater than 4.5), the protease enzymes are not activated.[3] This prevents the body from breaking down the protein into amino acids. If the protein cannot be digested appropriately, it may be recognized by the GI immune system as foreign, triggering an inflammatory response throughout one’s body. Some would argue that this might explain the rise in the incidence of inflammatory conditions, such as eosinophilic esophagitis, [4][5] and food protein intolerances. Within five days or less of taking a proton pump inhibitor (PPI) medication, pH will rise to greater than 4.0.[6]
This may also explain why there is a significant intolerance to the cow’s milk protein, casein, in many people, and why they often feel better drinking raw milk. When milk is pasteurized, the heating process destroys bacteria and enzymes that help break down the casein protein. Because casein is not broken down, it may be seen by the immune system as a foreign protein and trigger inflammation.[7]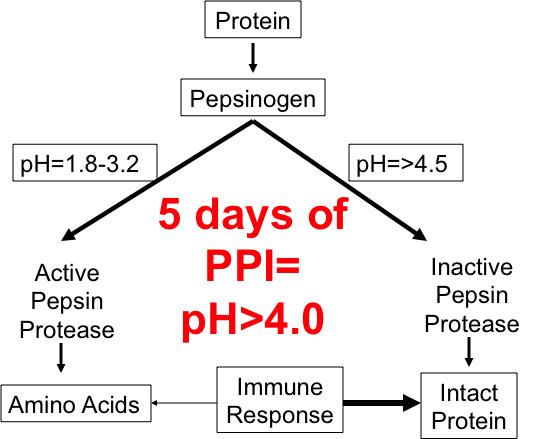 Figure 3. Difference in Protein Breakdown with Different Gastric pH Levels.The number of studies showing the potential harm of prolonged acid suppression is growing. Every prescriber of these medicines should be aware of the potential complications of persistent acid suppression.
Figure 3. Difference in Protein Breakdown with Different Gastric pH Levels.The number of studies showing the potential harm of prolonged acid suppression is growing. Every prescriber of these medicines should be aware of the potential complications of persistent acid suppression.
Every prescriber of proton pump inhibitors (PPIs) should be aware of the potential complications of reducing acid and raising pH long-term.
One of the important risks of chronic acid suppression is that it allows bacteria to grow where it should not. Acid suppresses much of the bacterial growth in the small intestine, and small intestinal bacterial overgrowth (SIBO) can result if acid is suppressed long-term.
Ideally, bacteria should populate the large, but not the small, intestine as this can have a significant impact on the health of the individual.
Potential Harms of Chronic Acid Suppression
The following information connects the known complications and mechanism of action associated with chronic acid suppression. [8]
Infection Risk
Community-acquired pneumonia
In a large cohort study, the use of acid suppressive drugs (both H2 blockers and PPIs) was associated with an increased risk of community-acquired pneumonia. This risk translated to one case of pneumonia per 226 patients treated with PPIs and 508 persons treated with H2 blockers. [9]
Gastroenteritis and community-acquired pneumonia in children
In one study, two months use of ranitidine or omeprazole in children 4-36 months of age resulted in significant increases in gastroenteritis (47% vs. 20%) and community-acquired pneumonia (12% vs. 2%) versus controls. [10]
C.difficile colitis
In a large population based case-control study, chronic use of histamine receptor antagonists and proton pump inhibitors increased the risk of community-acquired Clostridium difficile infection by 8% vs. 4% and 23% versus 8% respectively compared to controls. [11]
Small bowel bacterial overgrowth (SBBO)
Infectious Risk: This study found that 26% of children treated with at least one month of a PPI had evidence of SBBO by hydrogen breath testing. [12]
Malabsorption Risk
Calcium malabsorption/hip fracture
Calcium is a relatively insoluble molecule and tends to bind to fiber. Thus, it needs HCl to dissolve and release it from its fiber carrier. [13][14][15][16]
B12 malabsorption/neurological dysregulation
HCl is needed to release vitamin B12 from its carrier protein so that it can then combine with intrinsic factor to later be absorbed in the terminal ileum. [17][18]
Iron malabsorption/anemia
HCl releases nonheme iron from its fiber carrier and then dissolves it. [19][20]
Magnesium malabsorption/cardiac arrhythmia
Long-term use of PPI is associated with greater risk. Magnesium levels can return to normal after one week of stopping PPI. Can cause cardiac arrhythmia, tremor and seizures if low. [21]
Establishing the Microbiome
The volume of research on the types of bacteria that populate our bodies is growing quickly. Some types of bacteria are helpful, and others are potentially harmful. When the balance of good and bad bacteria tips toward the unhealthy side, it is called dysbiosis. Probiotics are beneficial bacteria and prebiotics are the food for these bacteria. Perhaps the most important and common prebiotic is fiber. Fiber not only feeds the bacteria, but it also stimulates the production of mucus, which the bacteria need to grow. Other prebiotic foods include those with strong smells, such as garlic, onions, and asparagus. In general, fruits (particularly bananas), whole grains, and vegetables are good prebiotics.
We are finding that the bacteria in our gut have many beneficial functions. Some of these include[22] the following:
- Influencing the expression of glycoproteins on the surface of enterocytes, which foster communication with other immune cells
- Helping to promote (as does fiber) mucin production, enhancing the mucous layer that lines the GI mucosa
- Aiding in the defense of potentially pathogenic bacteria by the direct release of anti-microbial peptides
- Helping to regulate the release of inflammatory cytokines such as interleukin, tumor necrosis factor and interferon
- Sharing genetic material with the host to facilitate digestion of specific food groups[23]
The research on using specific strains of probiotics is growing quickly, and the many benefits found highlight the importance of this synergistic human-microbiome relationship.
Summarized below are some of the more promising clinical studies of probiotics.
Conditions and Probiotics
The following information is a list of conditions that have the strongest evidence in support of using probiotics for prevention and treatment.
Prevention of Upper Respiratory Infection (URI) in children
- Bacteria strains: Lactobacillus acidophilus +/- Bifidobacterium animalis
- Benefits seen with Lactobacilli alone and combined with Bifidobacteria given twice daily for 6 months [24]
Prevention of colic, reflux, and constipation in infants
- Bacteria strains: Lactobacillus reuteri DSM 17938 during the first 3 months of life
- Mean duration of crying time was 38 minutes for probiotic group vs. 71 minutes in placebo group (P < .01) [25]
Prevention of diarrhea in preschoolers
- Bacteria strains: Lactobacillus reuteri DSM 17938 1 x 108 CFU given daily for 3 months
- Not only reduces the incidence and duration of diarrhea but also reduced the incidence of URI [26]
Prevention of atopic dermatitis in infants
- Bacteria strains: Lactobacillus rhamnosus strain GG (Culturelle)
- Benefits seen when this was given to mothers prenatally and then to their offspring for 6 months. The benefit persisted at 4-year follow-up. [27]
Ability to store fat
- Bacteria strains: Bacteria from human twins (overweight and normal weight) were transplanted into sterile mice.
- Bacteria from overweight twin caused mice to gain more weight. Eating a high-fiber, low-fat diet negated the effect. [28]
Irritable bowel syndrome
- Bacteria strains: Bifidobacterium infantis 35624
- The dose of 108 CFU worked best in this study of women. The available data supports Bifidobacteria to be more effective than Lactobacillus at reducing gas and bloating. [29][30]
C. difficile colitis
- Bacteria strains: Donor stool infusion
- Significant benefits have resulted in this becoming the therapy of choice for drug-resistant cases. [31]
- Single-strain probiotic (no clear species recommended); >50% reduction in colitis in hospitalized individuals when started within 2 days of antibiotics.[32]
Some of the best research regarding the benefit of probiotics has been conducted with infants and children. The environment and types of bacteria the newborn is exposed to is important. Once their microbiome is established, it may be difficult to alter.[33] While a baby is in utero, it has a sterile intestine. At the time of delivery, the baby is exposed to bacteria as it travels through the birth canal (mainly Lactobacilli) and with breast-feeding and skin contact with the mother (mainly Bifidobacteria). The type of bacteria the baby is exposed to is different if he/she is born by cesarean section or bottle-fed.[34] Also, if a mother is group B Streptococcus (GBS) positive, she will receive peripartum antibiotics that change the vaginal bacterial growth. There are some early findings suggesting that birth via cesarean section is associated with a higher incidence of cow’s milk allergy and atopy,[35][36][37] although the data is conflicting.[38]
While the mode of delivery and breast- versus bottle-feeding influences the unique microbiome early in life, the type of diet one eats has the biggest effect later in life. Eating a low-fat, high-fiber diet rich in vegetables and fruit is associated with more diversity of the human microbiome.[39] Eating a high-fiber, plant-based diet has also been associated with a higher growth of Bacteroidetes and Bifidobacteria. These bacteria reduce the efficiency of energy absorption in the gut and are associated with less obesity.[40][41]
Gut bacteria use soluble fiber (the type that absorbs water) to produce short-chain fatty acids (SCFAs) through fermentation. One of these SCFAs, butyrate, is the main source of energy for colonocytes. Colonocytes need SCFAs to repair themselves and reduce inflammation. The SCFAs acetate, propionate, and n-butyrate, can be measured in stool tests (comprehensive diagnostic stool analysis) to assess the amount of bacterial fermentation. Adequate production of SCFAs has been associated with reduced risk of inflammatory bowel disease and colon cancer.[42] Some clinicians use butyric acid enemas to treat ulcerative colitis. Both overuse of antibiotics and poor nutrition (including a low-fiber diet) can reduce the levels of SCFAs in the colon, because both reduce the healthy fermentation that normally occurs due to the interactions of fiber, food, and the microbiome.
Fermentation from fiber and gut bacteria produces short-chain fatty acids that help repair the gut lining and reduce inflammation.
To see if this intestinal fermentation can influence conditions beyond the colon, Trompette and colleagues showed that when mice were fed a high- vs. low-fiber diet, the high-fiber group produced more SCFAs. These SCFAs were associated with less inflammation and airway hyper-reactivity in the lungs when compared to the low-fiber group. The high-fiber diet also resulted in the growth of bacteria in the Clostridium genus, and they produced more SCFAs. In summary, fiber in the diet not only reduced inflammation and mucus in the lungs, but also promoted the growth of bacteria that were more likely to produce these anti-inflammatory effects.[43][44] For more information on probiotics, refer to the “Promoting a Healthy Microbiome with Food and Probiotics” Whole Health tool.
Summary
As indicated by reviews cited above, research data supports the fact that the GI tract has many influences that modulate the balance between health and disease. This balance starts at the time of birth and is influenced throughout the life cycle by nutrition, medications, emotions, and many other components of the Circle of Health.
Let us turn our attention back to Jake’s situation and see how we can use the Circle of Health and specific therapeutic strategies to help Jake achieve his health goals.
Self-Care
Moving the Body
Jake feels pretty good about his physical health. We may want to encourage him to maintain his fitness. Activity is one of the most important triggers of regular bowel evacuation.
Movement and aerobic activity are powerful tools that use the autonomic nervous system to improve GI function. Movement helps promote peristalsis while also reducing emotional stress, allowing the parasympathetic nervous system to have a greater influence than the sympathetic nervous system. Most GI function works optimally when parasympathetic tone predominates. The fight or flight process (sympathetic stimulation) reduces blood supply to the gut. This reduces pancreatic enzyme secretion, which inhibits adequate digestion. This is why eating on the run or eating while under stress can cause havoc on digestive function. Stress also increases cortisol, a steroid that causes the body to store energy as fat.
Mild intensity physical exercise has been found to increase gas clearance and reduce bloating.[45] Women who are more physically active tend to have fewer and less-severe IBS symptoms.[46] One small randomized controlled trial (RCT) showed that increasing physical activity had symptomatic benefits in those with constipation-predominant IBS.[47] Regular exercise helps reduce symptoms of gastroesophageal reflux disease, but vigorous exercise may worsen symptoms.[48] Despite these demonstrated benefits of exercise on GI symptoms, research does not support alternative exercise recommendations for those with IBS versus the general population.
With Jake’s frequent loose stools, we would recommend that he maintain his current exercise and movement program, without increasing activity at this time, as this may promote more peristalsis, increasing his stool frequency.
Food and Drink
Jake does not have much fiber in his diet. He reports eating lots of “white foods,” and he has noticed that beer seems to exacerbate his symptoms.
The first-line approach to Jake’s diet should be to optimize his adherence to guidelines for general, healthy eating. Given that most Americans do not adhere to general dietary guidelines, these recommendations should serve as a starting point. Further, those with IBS may be particularly sensitive to excessive consumption of alcohol, spicy foods, caffeine, and dietary fat, and inadequate consumption of hydrating fluids.[49]
In determining if a food group could be exacerbating symptoms, we often ask if there are any foods that a person craves or eats frequently. It is thought that increased intestinal permeability might allow the foods we crave the most to cross the gut barrier and stimulate enteric endorphins; this may actually cause one to crave the very foods that may be triggering symptoms. Jake likes “white foods” such as bread and pasta. He also feels that his diarrhea is worse when he drinks beer. Both white foods and beer are rich in gluten. It is possible that the long course of antibiotics he required disrupted the integrity of his intestinal barrier, making his gut immune system sensitive to gluten. In other words, he may have non-celiac gluten sensitivity, which is different than having celiac disease.
Since celiac disease (CD) has long-term health consequences (GI malignancy, osteoporosis, etc.), it is important to accurately diagnose this condition. Many people with non-celiac gluten sensitivity (NCGS) receive the diagnosis of CD inappropriately. CD requires strict gluten restriction, while people with NCGS often do fine if they keep gluten load to a minimum while taking measures to improve their GI health. They can titrate the amount of gluten they eat based on the severity of their symptoms.
Celiac Disease and Non-Celiac Gluten Sensitivity
Celiac disease (CD) presents differently than non-celiac gluten sensitivity (NCGS).[ref id=50][51] There main questions help us distinguish CD from NCGS.
- Question #1. Are CD serology tests positive? Check tTG IgA (transglutaminase IgA) and DGP (deamidated gliadin peptide antibodies). If positive, CD is likely.
- Question #2. Are there signs and symptoms of malabsorption? These include weight loss, diarrhea, and abnormal nutrient testing indicating low vitamin D, iron, B12 and/or zinc. (Low vitamin D and iron were most predictive for CD). Note: Take care with patients on chronic acid suppression medications, as they can have malabsorption as well.
- Question #3. Is there a family history of CD or a personal history of autoimmune disease? (CD is more likely associated with other autoimmune disease).
If all these queries are negative and the patient feels better on a gluten-free diet, he/she has NCGS, and endoscopy with biopsy is unnecessary.
If serology is negative, but questions #2 and/or #3 are positive, order HLA genetic testing. If negative, the patient has NCGS. If positive, order endoscopy with biopsy.
Remember, serology testing needs to be done while a person has been eating gluten. Otherwise, there are risks of falsely negative results. Although recommendations vary, many experts recommend that gluten is eaten for 8 weeks to stimulate antibodies prior to CD testing. If your patient cannot tolerate gluten, consider genetic HLA DQ2/DQ8 genetic testing. If this is negative, the person likely has NCGS. Table 1 for a comparison between CD and NCGS.
Table 1. Celiac Disease versus Non-Celiac Gluten Sensitivity
| Celiac | Non-Celiac Gluten Sensitivity |
|---|---|
| More diarrhea | More constipation |
| Presents later in life | Presents earlier in life |
| Positive serology (tTG IgA, DGP) | Negative serology (tTG IgA, DGP) |
| Symptoms of malabsorption (weight loss, diarrhea) are more common | Symptoms of malabsorption (weight loss, diarrhea) are less common |
| Order genetic testing (HLA DQ2/DQ8) if serology is borderline with symptoms of malabsorption. If negative then likely NCGS. | Not needed if serology is negative |
| Family history | No family history |
| Other auto-immune disease | No other auto-immune disease |
| Consider endoscopy and biopsy | Endoscopy and biopsy rarely needed |