Minneapolis VA Health Care System Research Service
New Investigator Orientation: Research Approval
Investigator Orientation: Overview | Required Information | Research Approval | Investigator Responsibilities | Research Funding | Hiring Personnel
The research approval and oversight process at VA is different from the structure used in nearly all other research institutions. In VA, every research project - including data analysis only studies, or studies that are exempt from IRB oversight - must undergo review and approval from the Research & Development Committee (RDC) as well as all applicable subcommittees. Further, every research project must also be approved by the Associate Chief of Staff for Research (ACOS) before any work can begin.
We highly recommend that all new investigators read and understand the information presented here. This page explains in detail the steps involved in obtaining approval to conduct VA research. We also recommend that new investigators review the information on our program's RDC, as well as the RDC subcommittees (IRB, IACUC, and SRS) in charge of human subjects protection, animal subjects protection, and research safety, respectively.
Study Approval Process
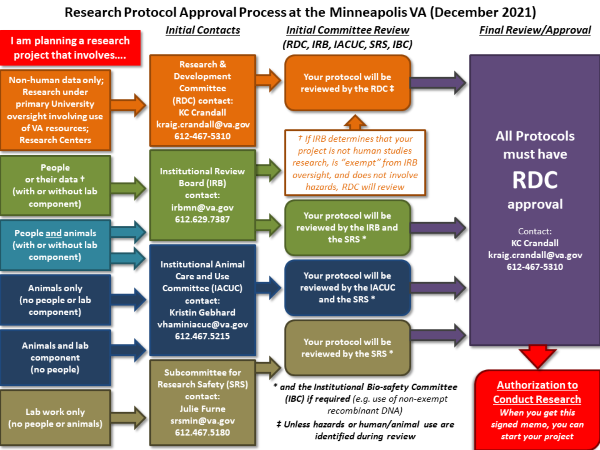
VA requires that all research studies (including nonfunded projects and systematic reviews) must be submitted for review and approval prior to initiating any research. The Minneapolis VA uses an electronic protocol management system (IRBNet) for all research protocols. If you are initiating a new project, or being added to an existing project, you will need to create or affiliate an account in IRBNet by following the IRBNet account creation instructions. New protocols submitted in IRBNet are reviewed and approved by the RDC and all relevant subcommittees. The 3 subcommittees are:
- Institutional Review Board (IRB), responsible for safety and ethics of research involving human subjects
- Institutional Animal Care and Use Committee (IACUC), responsible for safety and ethics of animal subjects research
- Subcommittee on Research Safety (SRS), responsible for the safety of personnel conducting research
Projects that do not involve safety concerns, animals, or are exempt from IRB review might require a review by the RDC only. All projects are required to be reviewed annually by the initial committee(s) unless a protocol amendment removes the portion of the protocol that prompted that committee's review.
Each Investigator and Co-Investigator on a VA research project is required to complete a Research Financial Conflict of Interest (COI) Statement annually, for each project in which they participate. This COI statement is required by the VA Office of General Counsel. This form must be filed when initially requesting approval of a new study, and updated statements must be filed annually thereafter until the study is closed. One key area in which conflicts of interest may arise is participating in or consulting on non-VA projects. For guidance in handling these situations, refer to the "Serving as a co-investigator or consultant" FAQ.
Authorization to Conduct Research
It is critical to note that approval from the RDC or subcommittee does not constitute permission to initiate research activities in VA. Final approval and permission to begin research is indicated by the Authorization to Conduct Research (ACR) letter, signed by the ACOS. After RDC approval, any outstanding administrative concerns must be satisfied before the ACR will be issued to the investigator.
Administrative issues that can delay issuance of the ACR include failure to complete required training, or failure to provide investigator information required by our program. If the proposed research involves a contract or cooperative research and development agreement (CRADA; commonly required in industry-funded studies), the ACR will not be issued until after the contract/CRADA has been finalized.
Applicable policy and guidance:
- RDC-001 "Research and Development Committee"
- RDC-011 "ACOS Approval Prior to Initiation of Research"
- RDC-021 "Managing Potential Conflicts of Interest"
- RDC-013 "Research Agreements Facilitated by the VA-NPC"



















