Minneapolis VA Health Care System Research Service
New Investigator Orientation: Research Funding
Investigator Orientation: Overview | Required Information | Research Approval | Investigator Responsibilities | Research Funding | Hiring Personnel
Obtaining research funding is critical to the success of our program. For clinicians-investigators, securing funding may be necessary to negotiate for protected research time. For non-clinician investigators, securing VA funding is essential to maintaining the 5/8ths VA-paid appointment required to remain eligible for VA grant submission. This page is intended to provide a broad overview to help investigators determine the steps involved in securing funding for a planned research study. Detailed instructions (if available), references, and relevant policy are linked below.
Funding Opportunities
Researchers in our program typically secure funding through the VA, through other Federal agencies such as the Department of Defense (DOD) or National Institutes of Health (NIH), through cooperative research agreements with pharmaceutical and medical device companies, or through grants issued by private foundations. For investigator-initiated grants, you should start by finding a Funding Opportunity Announcement (FOA) or Request For Application (RFA) of interest.
Information for VA FOA/RFAs and Program Announcements are located on the VA Office of Research & Development (ORD) Intranet site at http://vaww.research.va.gov/funding/rfa.cfm (copy and paste this link into your browser).
Information for DOD RFPs can be found on the Congressionally Directed Medical Research Programs (CDMRP) website.
Information for NIH RFPs can be found on the NIH Grants & Funding website.
Information for research funding opportunities available through private industry or foundations may be available through the VA-affiliated non-profit corporation (VA-NPC), Center for Veterans Research & Education (CVRE).
Investigators should note that regardless of where research funding is obtained, all funds used to support VA research at the Minneapolis VA must be administered through the VA, CVRE, or the University of Minnesota.
Submitting a Grant Application
The process for submitting a grant application is dependent upon which organization will administer the funding should the application be successful. Grant applicants can find frequently required institutional information (legal name, unique entity identifier, authorized signatories, and applicable assurance numbers) on the Institutional Information page.
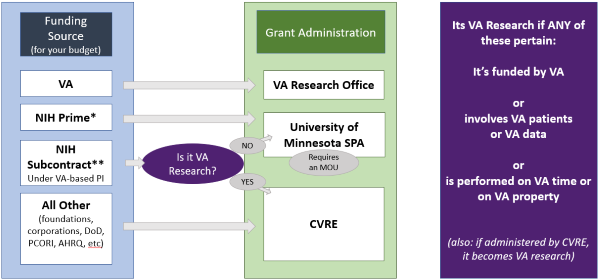
VA Grants
VA research funds may only be disbursed to and administered by a VA facility. Non-VA institutions may be included in a VA grant, but no funds or subcontracts can be directly awarded to these non-VA institutions. VA grants have eligibility requirements that differ based on the ORD service funding the award. In general, VA grants may only be awarded if the Principal Investigator (PI) has employment status and activities that demonstrate a primary professional commitment to VA. To be eligible for VA grants, a VA PI must have at least 5/8ths VA-paid effort, or be brought to 5/8ths by accepting the funds. While clinicians are eligible to apply for all VA FOA/RFAs, non-clinicians may need to meet additional eligibility requirements. For additional guidance, contact the Deputy ACOS.
VA grants are offered by four ORD service lines: Biomedical Laboratory R&D (BLRD), Clinical Science R&D (CSRD), Health Services R&D (HSRD), and Rehabilitation R&D (RRD). At our facility, HSRD applications are submitted by the Center for Care Delivery and Outcomes Research (CCDOR), our HSRD Center of Innovation (COIN). All other VA grants (BLRD, CSRD, RRD, and CSP) are submitted through the Research Office.
The VA grant submission process for the Minneapolis VA is discussed in detail in the Submitting a VA Grant FAQ.
Non-VA Grants
Grants from the DOD are submitted through and administered by CVRE. Grants from other Federal agencies, such as NIH, are administered either by CVRE or through your affiliated department at the University of Minnesota, with subawards through CVRE as appropriate. Funding from sources other than Federal agencies, such as industry-sponsored studies or studies funded by private foundations, should always be administered through CVRE.
For assistance with submissions through the University of Minnesota, investigators affiliated with the Medical School should refer to the UMN Medical School Pre-Award and Proposal Development Services website. This new pre-award site focuses on Medical School faculty with VA Medical Center joint appointments who conduct research via the University. It includes memorandum of understanding guidance, FAQs, templates, and sample language for proposal development. For assistance, contact Erin Brudvik, VA Medical Center/Medical School Research Manager.
University of Minnesota affiliates outside of the Medical School should contact their departmental Sponsored Projects Grants & Contract Officer.
For assistance with submissions through CVRE, refer to the CVRE website.
Protected Research Time
Investigators with clinical appointments are expected to ensure clinical care coverage is prioritized. However, VA guidance does recommend ensuring protected research time is provided for clinicians conducting research activities. For recommended clinician protected time information, refer to the information in "Guidance on Protected Time for Various Research Activities". Clinician-investigators should keep in mind that this document consists of guidance, not a guarantee, of protected time. You must negotiate with your ICC to obtain time dedicated to research activities. While some ICCs make protected time possible, clinical pressures and/or staffing shortages may prevent any agreement for protected time in other ICCs.
Clinicians on mentored VA Career Development Awards (CDAs) are allowed to request up to 6/8ths protected research time for the duration of the award, with salary for this time to be supported by the grant. The remaining effort (up to 8/8ths) is to be reserved for clinical care duties. The exact percentage of protected time is expected to be negotiated with the clinical service in which the awardee works and must be included as part of the grant application via a signed support letter from the clinical care chief or department head.
Salary Support
Clinicians are prohibited from requesting salary on nearly all VA grants. VA Career Development Awards are the exception. As stated above, clinician CDA recipients can request salary for up to 6/8ths protected research time, with salary from their ICC covering clinical time for the remaining effort (up to 8/8ths). Non-clinician investigators can request up to 100% of their salary from a VA grant. Some VA grants limit salary requests to the actual percent effort listed in the application, and salary support in some cases is not excluded from the budget cap.
Investigators cannot exceed 100% (8/8ths) VA-paid effort. For grants administered by CVRE, effort on the award is considered to be VA effort. Full-time VA personnel thus cannot accept salary through CVRE. For grants administered by the University of Minnesota, investigators can request partial salary support, but must file a Memorandum of Understanding (MOU) stipulating the breakdown of effort and salary support between VA and the university to prevent instances of dual compensation (see NIH Grants Policy Statement 17.3, VA-University Relations). To initiate or update a MOU, contact Erin Brudvik, VA Medical Center/Medical School Research Manager.
Budget and Finance
Contact the Research Budget Analyst for assistance in preparing and managing your budget for VA grants. For routine purchases using VA funds, submit requests to the Research purchase card holders via email by sending a completed General Medical Ordering Form to VHAMINResearchOrders@va.gov. For larger purchases, a contract may be required. Contact the Research Budget Analyst for assistance.
Remember that you cannot incur a charge before a purchase order or contract is in place: VA financial regulations define this as an unauthorized commmitment of Federal funds. Personnel who create an unauthorized commitment may be held personally liable for the incurred cost. You should always contact the Budget Analyst or VA purchase card holder for assistance before you take any action involving VA funds.
Applicable policy and guidance:
Next: Hiring Personnel



















